��Ŀ����
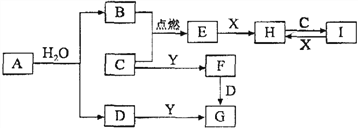
����Ŀ���ϳɰ����Ȼ�ѧ����ʽΪN2(g)+3H2(g)![]() 2NH3(g) ��H= - 92.4 kJ/mol���ֽ�1mol N2(g)3mol H2(g)����һ�ݻ�Ϊ2L���ܱ������У���500���½��з�Ӧ��10minʱ�ﵽƽ�⣬NH3���������Ϊ
2NH3(g) ��H= - 92.4 kJ/mol���ֽ�1mol N2(g)3mol H2(g)����һ�ݻ�Ϊ2L���ܱ������У���500���½��з�Ӧ��10minʱ�ﵽƽ�⣬NH3���������Ϊ![]() ������˵������ȷ���ǣ� ��
������˵������ȷ���ǣ� ��
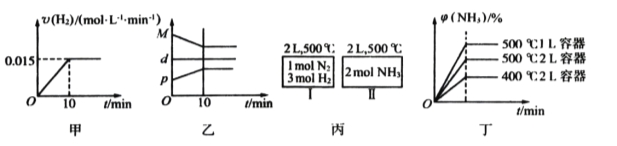
A�����ﵽƽ��ʱ�������ϵ�ų�9.24kJ��������H2��Ӧ���ʱ仯������ͼ����ʾ
B����Ӧ�����У��������ƽ����Է�������ΪM����������ܶ�Ϊd���������ѹǿΪp�����߹�ϵ��ͼ��
C����ͼ����ʾ������I��II�ﵽƽ��ʱ��NH3���������Ϊ![]() ��������I�ų�����������II��������֮��Ϊ92.4kJ
��������I�ų�����������II��������֮��Ϊ92.4kJ
D������ʼ��������Ϊ1 mol N2��3 mol H2���ڲ�ͬ�����´ﵽƽ��ʱ��NH3����������仯��ͼ����ʾ
���𰸡�C
��������
���������A���ﵽƽ��ʱ�ų�������Ϊ9.24kJ�����Ȼ�ѧ����ʽ��֪�μӷ�Ӧ�����������ʵ���Ϊ0.3mol��10min��������ƽ������Ϊ0.015mol/��Lmin������Ӧ����ӦΪ�ɸߵ�����A����B�������������������䣬������������䣬���������ܶ�Ϊ��ֵ���淴Ӧ���С������������ʵ�����С����������ƽ����Է�����������������ѹǿ������B����C�����º����£��������а���ѧ������ת��ΪN2��H2���ɵ�N21mol��H23mol������������������Ϊ��Чƽ����ƽ��ʱ�����ڶ�Ӧ���������ʵ�����ȣ�����I�ų�����������II��������֮��Ϊ92.4kJ��C��ȷ��D������ѹǿƽ��������Ӧ�����ƶ�������������������������¶�ƽ�����淴Ӧ�����ƶ���ƽ��ʱNH3�����������С��D����ѡC��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ������������SO2����������������NOx���������ǻ�����ѧ�о����ȵ㡣
��1���������������Ļ���������______________________��
��2��һ��ѡ���Դ�������NO2����ԭ��Ϊ��6NO2��8NH3 ![]() 7N2��12H2O
7N2��12H2O
�� ������Ӧ�б���ԭ��Ԫ����_________����Ԫ�ط��ţ�����Ӧ��ÿת��3mol���ӣ����ɱ�״����N2�����Ϊ____________��
�� �����ٷɻ��ŷŵ�β����ƽ������NOx����Ҫ��Դ�������ƻ����������Ҫ����Ϊ��
�� ��O3![]() O��O2�� �� ��NO��O3��NO2��O2 �� �� NO2��O�� NO��O2
O��O2�� �� ��NO��O3��NO2��O2 �� �� NO2��O�� NO��O2
������Ӧ��NOx�����������_____________��
��3���±��г���2��ȼú����������ԭ����
������ | �ð�ˮ��SO2ת��ΪNH4HSO3����������(NH4)2SO4 |
������ | ���������Ƚ�������Ҫ�ɷ�CO��CH4��H2����SO2�ڸ����»�ԭ�ɵ����� |
�����������ð�ˮ����ȼú�����е�SO2ת��ΪNH4HSO3����������SO2��___________���ʣ�ѡ����ĸ��ţ���
A��Ư���� B�������� C����ԭ�� D������������
�ڷ������а�ˮ����ȼú������SO2�Ļ�ѧ��ӦΪ��2NH3+SO2+H2O=��NH4��2SO3
��NH4��2SO3+SO2+H2O=2NH4HSO3�������ȼú������SO2ȥ���ʵĴ�ʩ��______������ĸ����
A������ˮŨ�� B�����߷�Ӧ�¶�
C��ʹȼú�����백ˮ��ֽӴ� D��ͨ�����ʹHSO3-ת��ΪSO42-
�����÷�������������ҪԤ�ȳ�ȥȼú�����д�����CO2��ԭ����_________________�������ӷ���ʽ��ʾ����