��Ŀ����
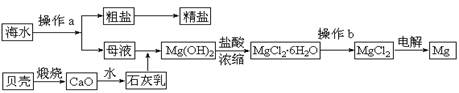
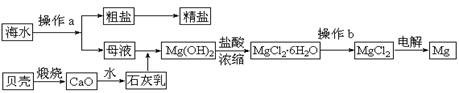
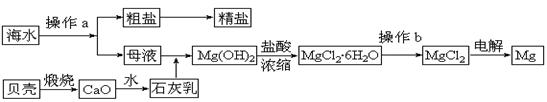
��ˮ��һ�ַḻ����Դ����ҵ�ϴӺ�ˮ�п���ȡ���������ʣ��㷺Ӧ��������������Ƽ��ȷ��档��ͼ��ij�����Ժ�ˮ��Դ�����ۺ����õ�ʾ��ͼ��
�ش��������⣺
(1)����ͼ�в���a������Ϊ ��
(2)��ҵ�ϴӺ�ˮ����ȡ��NaCl����������ȡ���꣬���Ҫ�������£���ʳ��ˮ����ͨ������A����ͨ������B����ַ�Ӧ����˵õ�����C����ҺD��������C���ռ����Ƶô��
������A��B��CO2��NH3��������AӦ�� ���ѧʽ����
����ҺD����Ҫ����NH4Cl��NaHCO3�����ʣ���ҵ��������ҺD��ͨ��NH3��������ϸСʳ�ο�������ȴ����������NaHCO3�ĸ���ƷNH4Cl���壬��ͨ��NH3��������
��
(3)þ��һ����;�ܹ�Ľ������ϣ�Ŀǰ������60%��þ�Ӻ�ˮ����ȡ��
����Ҫ��֤������ˮMgCl2�в���NaCl����IJ��������ǣ�
��
�ڲ���b���� ��Χ�н��У����ڿ����м��ȣ��������Mg(OH)Cl��д���йط�Ӧ�Ļ�ѧ����ʽ�� ��
�������ᾧ�������� 1�֣�
�Ƣ�NH3��1�֣�������NH4+��Ũ�ȣ������ڳ���ƽ��������NH4Cl�ķ������,��NaHCO3ת��ΪNa2CO3�����������������1�֣���2�֣�
�Ǣ��ò�˿պȡ��������,���ھƾ��ƻ���������,����ɫ�������,��֤��������ˮ�Ȼ�þ�����в����Ȼ��ơ���1�֣�������ɫ��ӦҲ���֣�
��HCl����������1�֣� MgCl2��6H2O == Mg(OH)Cl+HCl+5H2O��2�֣�
����:
���⽫���ƶ���þ�Ĺ�ҵ�Ʒ�����°��Ƽ�Ƚ����һ�𣬿�������֮����ת����ˮ�⡢��ѧ�����֪ʶ���ڲ���Ļ����Ͻ������������ǽ��ո߿���ѧ�����й�ҵ����ͼ�⡱���ص㡣
��1�� ��ˮ��ɹ�εĻ���ԭ��������ȥˮ��ʹ�νᾧ������
��2�� ��ͨ��NH3��ˮ���ܽ�Ⱥܴ�������ˮʹ��Һ�ʼ��ԣ��Ӷ�������CO2���ܽ�����ʹ����������ӡ���°��Ƽ�Ļ���ԭ����NH3+NaCl+CO2+H2O=NaHCO3+NH4Cl.
��3�� �ټ���Na+����ѷ���������ɫ��Ӧ ��HCl���������������Ƿ�ֹMgCl2��6H2O�ڼ��ȹ����з���ˮ�ⷴӦ��

 ������ȫ�̼����ĩ���100��ϵ�д�
������ȫ�̼����ĩ���100��ϵ�д�