��Ŀ����
��ҵ�ϲ���SO2��N2��O2���������SO2������װ����ͼ15-17����Ӧ����װ�е�ĵ�����Һ��SO2��I2�����ķ�ӦΪ��N2��O2����I2��Ӧ����SO2+I2+2H2O====H2SO4+2HI����1�����������뷴Ӧ�ܺ������������ӵ�ˮ��������ڵ�_____________________�������д����Ļ�ѧʽ����
��2����Ӧ������Һ��ɫ��ʧ��û�м�ʱֹͣͨ�������õ�SO2����_____________��ѡ���ƫ�ߡ���ƫ�͡�����Ӱ�족����
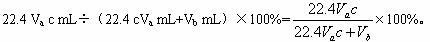
ͼ15-17
��3����Ӧ���ڵĵ�ĵ�����ҺҲ������_______________________���棨��д�������ƣ���
��4��������Һ���ΪVa mL��Ũ��Ϊc mol��L-1��N2��O2�����ΪVb mL��������Ϊ��״���µ����������c��Va��Vb?��ʾSO2������ٷֺ���Ϊ_______________��
��5��������װ�ø�Ϊ����ʵ��װ�ã��������⣬����ѡ�õ�����Ϊ_____________��ѡ���������ı�ţ���
a.�ձ� b.�Թ� c.���ƿ d.����ƿ e.��Ͳ f.������ g.˫����
��������1�����������뷴Ӧ���У�����SO2��I2������Ӧ��SO2+I2+2H2O====2H2SO4+2HI�����������壬ʣ����������N2��O2�������������ӵ�ˮ���������N2��O2���������
��2����Ӧ������Һ��ɫ��ʧʱ��SO2�뷴Ӧ����������I2ǡ�÷�Ӧ����û��ʱֹͣͨ������δ��Ӧ��SO2����Ҳ��ˮ�������ܣ�ʹN2��O2������ӣ����SO2�ĺ������͡�
��3����ĵ�����Һ�������ǵ����е�I2��ȫ��SO2��ԭʱ����Һ����ɫ������ɫ����ɫ��ָʾ�յ�ġ���������SO2���巴Ӧ�����ʣ��ڴﵽ�յ�����ɫ�ı�ʱ��ɴ����ĵ�����Һ����KMnO4��Һ����ˮ�ȡ�
��4����������Ӧ֪SO2��������ʵ������ڵ�����ʵ���Va��10-3 L��c mol��L-1=Vac��10-3 mol�������ΪV (SO2)=Vac��10-3 mol��22 400 mL��mol-1=22.4 Vac������SO2���������Ϊ��![]()
��5��������װ�ÿ�֪��Ӧ�����ܱ����������ã���˿����Թܡ����ƿ���棬����ȡ�����������������������⣬�����ò����������ų�Һ������ȷ�������ù��ƿ��˫��������Ͳ���������ܡ��ʸ�Ϊ����װ����ѡ����Ϊbceg��beg��ceg��
�𰸣���1��N2��O2 ��2��ƫ��
��3���������������Һ����ˮ
![]()
��5��bceg��beg��ceg

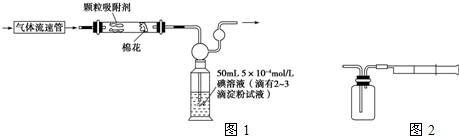






 ��ͬѧ���������ͼ��ʾ��ʵ��װ�ã��ⶨij�ؿ�����SO2�ĺ�����
��ͬѧ���������ͼ��ʾ��ʵ��װ�ã��ⶨij�ؿ�����SO2�ĺ����� ����ͬѧ��������ͼ����װ�òⶨ�����е�SO2������ȷ��ȡһ�������5��10-4mol/L�ĵ���Һ��ע����ͼ��ʾ���ƿ�У���2��3�ε���ָʾ������ʱ��Һ����ɫ����ָ���IJⶨ�ص������ÿ�γ���100mL��ֱ����Һ����ɫȫ���ʾ�Ϊֹ����¼����������n����
����ͬѧ��������ͼ����װ�òⶨ�����е�SO2������ȷ��ȡһ�������5��10-4mol/L�ĵ���Һ��ע����ͼ��ʾ���ƿ�У���2��3�ε���ָʾ������ʱ��Һ����ɫ����ָ���IJⶨ�ص������ÿ�γ���100mL��ֱ����Һ����ɫȫ���ʾ�Ϊֹ����¼����������n����