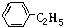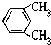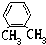��Ŀ����
15����A��һ����Ҫ�Ļ�������ԭ�ϣ��������������Է�������Ϊ28����10ͼ����AΪԭ�Ϻϳ�ҩ���м���E����֬K��·��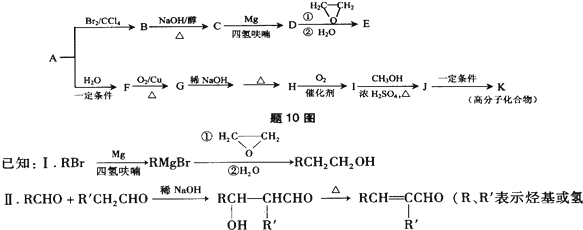
��1��A�й����ŵĽṹ��ʽ��
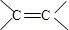 ��
����2��B��C�Ļ�ѧ����ʽΪBrCH2CH2Br+NaOH$��_{��}^{��}$CH2=CHBr+NaBr+H2O��
��3��E�ķ���ʽΪC4H8O�����й���E��˵����ȷ����ac������ĸ��ţ�����R��R���ʾ��������ԭ�ӣ�
a����������Ʒ�Ӧb��������4��̼ԭ��һ����ƽ��
c��һ�������£�����Ũ�����ᷴӦd����CH2CHCH2OCH2CH3��Ϊͬϵ��
��4��д����������������E��һ��ͬ���칹�壺��CH3��2CHCHO��
a�����л����������Ƶ�Cu��OH��2������Ӧ��b���˴Ź���������3��壬���ֵΪ6��1��1
��5��G��H�漰���ķ�Ӧ�����мӳɷ�Ӧ����ȥ��Ӧ��
��6��I�ķ���ʽΪC4H6O2����ṹ��ʽΪCH3CH=CHCOOH��
��7��J��K�Ļ�ѧ����ʽΪ
 ��
��
���� ��A��һ����Ҫ�Ļ�������ԭ�ϣ�A����Է�������Ϊ28��ӦΪCH2=CH2������ת����ϵ����Ӧ������֪��BΪBrCH2CH2Br�������Ϣ���֪CΪCH2=CHBr��DΪCH2=CHMgBr��EΪCH2=CHCH2CH2OH��A��ˮ�����ӳɷ�Ӧ����FΪCH3CH2OH��GΪCH3CHO������Ϣ���֪HΪCH3CH=CHCHO��IΪCH3CH=CHCOOH��JΪCH3CH=CHCOOCH3��KΪ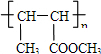 ���ݴ˴��⣮
���ݴ˴��⣮
��� �⣺��A��һ����Ҫ�Ļ�������ԭ�ϣ�A����Է�������Ϊ28��ӦΪCH2=CH2������ת����ϵ����Ӧ������֪��BΪBrCH2CH2Br�������Ϣ���֪CΪCH2=CHBr��DΪCH2=CHMgBr��EΪCH2=CHCH2CH2OH��A��ˮ�����ӳɷ�Ӧ����FΪCH3CH2OH��GΪCH3CHO������Ϣ���֪HΪCH3CH=CHCHO��IΪCH3CH=CHCOOH��JΪCH3CH=CHCOOCH3��KΪ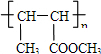 ��
��
��1�������Ϸ�����֪AΪCH2=CH2�����еĹ�����Ϊ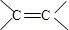 ��
��
�ʴ�Ϊ��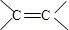 ��
��
��2��BΪBrCH2CH2Br��CΪCH2=CHBr��B����C�ķ���ʽΪBrCH2CH2Br+NaOH$��_{��}^{��}$CH2=CHBr+NaBr+H2O��
�ʴ�Ϊ��BrCH2CH2Br+NaOH$��_{��}^{��}$CH2=CHBr+NaBr+H2O��
��3��EΪCH2=CHCH2CH2OH������C=C���ܷ����ӳɷ�Ӧ������-OH������Na������Ӧ������������CH2=CHCH2OCH2CH3�ṹ��ͬ������ͬϵ�������ֻ��3��Cԭ����ͬһ��ƽ���ϣ�
�ʴ�Ϊ��ac��
��4��E��ͬ���칹�壬������������a�����л����������Ƶ�Cu��OH��2������Ӧ��˵����ȩ����b���˴Ź���������3��壬���ֵΪ6��1��1�������������칹��Ϊ��CH3��2CHCHO��
�ʴ�Ϊ����CH3��2CHCHO��
��5������Ϣ���֪G��H�漰���ķ�Ӧ�����мӳɷ�Ӧ����ȥ��Ӧ��
�ʴ�Ϊ���ӳɷ�Ӧ����ȥ��Ӧ��
��6�������Ϸ�����֪IΪCH3CH=CHCOOH��
�ʴ�Ϊ��CH3CH=CHCOOH��
��7��JΪCH3CH=CHCOOCH3������̼̼˫�����ɷ����Ӿ۷�Ӧ����Ӧ�ķ���ʽΪ ��
��
�ʴ�Ϊ�� ��
��
���� ���⿼���л�����ƶϺͺϳɣ���Ŀ�Ѷ��еȣ�ע�����AΪ��ϩ��������Ϣ�Լ������ŵ�ת���������ƶ��������ʵ����࣬����ʱע���л�����֪ʶ���ۺ�Ӧ�ã�

 ÿ�α���ϵ�д�
ÿ�α���ϵ�д�| A�� | ±�ص��ʵ���������������7 | |
| B�� | ���ϵ��£�±��ԭ�ӵĵ��Ӳ����������࣬�뾶���μ�С | |
| C�� | ±�ص�����H2���ϵ����׳̶�ΪF2��Cl2��Br2��I2 | |
| D�� | ��F��I��ԭ�Ӻ˶��������ӵ������������μ�����ԭ�ӵõ����������μ�����Ԫ�صķǽ��������μ��� |
| A�� | һ����Na2CO3��������FeCl3 | B�� | һ����MgCl2��һ����NaCl | ||
| C�� | һ����MgCl2��������Na2CO3 | D�� | һ����MgCl2��������NaCl |
| A�� | ���ʷ�����ѧ�仯�������������仯 | |
| B�� | ���������仯�����ʱ仯���ǻ�ѧ�仯 | |
| C�� | ��һ��ȷ���Ļ�ѧ��Ӧ��ϵ�У���Ӧ������������������������������ͬ | |
| D�� | ��һ��ȷ���Ļ�ѧ��Ӧ��ϵ�У���Ӧ������������Ǹ���������������� |
| �� | �� | �� | �� | |
| �������и�Ԫ��ԭ�Ӹ����� | A��C=1��1 | B��A=1��2 | D��E=1��3 | B��E=1��4 |
��1���ĵ���ʽ
 �����Ľṹʽ
�����Ľṹʽ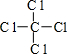
��2��EԪ�������ڱ��е�λ���ǵ������ڢ�A�壬��������ӵĽṹʾ��ͼΪ
 ������������ˮ���ﻯѧʽΪHClO4��
������������ˮ���ﻯѧʽΪHClO4����3������Ԫ���У��ȿ��������ᷴӦ���ֿ���������������Һ��Ӧ����Al����Ԫ�ط��ţ�����д����Ӧ�����ӷ���ʽ��2Al+6H+=2Al3++3H2����2Al+2OH-+2H2O=2AlO2-+3H2����
 ��
��

 ��
��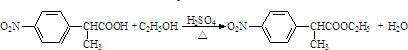 ��
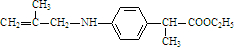
��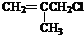 ����ͬ�����ŵ�ͬ���칹����10�֣���Ҫ����˳���칹��
����ͬ�����ŵ�ͬ���칹����10�֣���Ҫ����˳���칹��