��Ŀ����
����Ŀ������ԭ�������ĵ��������ֶ�����Ԫ��(����ĸx��h��ʾ)��ԭ�Ӱ뾶��Դ�С��������ۻ�����۵ı仯����ͼ��ʾ��
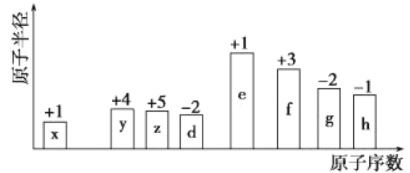
��ش��������⣺
��1��x�γɵ������ӵĽṹʾ��ͼΪ_____________ ��
��2�����zԭ���к�8�����ӣ�����ԭ�ӷ���Ϊ______________��
��3��f�ĵ��ʺ�e������������Ӧ��ˮ�������Ӧ�Ļ�ѧ����ʽΪ_______________��
��4���Ƚ�d��e�������ӵİ뾶��С��_______________�������ӷ��ţ�����������ʾ����
��5��g��h��Ԫ������������Ӧˮ�������Ը�ǿ����_____________�����ѧʽ��
��6������x��z��d����Ԫ�ص����ֻ�������кͷ�Ӧ�����ӷ���ʽ��__________________��
���𰸡�![]() 157N 2Al+2NaOH+2H2O==2NaAlO2+3H2�� O2- >Na+ HClO4 H++NH3��H2O==NH4++H2O
157N 2Al+2NaOH+2H2O==2NaAlO2+3H2�� O2- >Na+ HClO4 H++NH3��H2O==NH4++H2O
��������
��ͼ�еĻ��ϼۺ�ԭ�Ӱ뾶�Ĵ�С�������Ƴ�x��HԪ�أ�y��CԪ�أ�z��NԪ�أ�d��OԪ�أ�e��NaԪ�أ�f��AlԪ�أ�g��SԪ�أ�h��ClԪ�ء�
�ɷ�����֪��x��HԪ�أ�y��CԪ�أ�z��NԪ�أ�d��OԪ�أ�e��NaԪ�أ�f��AlԪ�أ�g��SԪ�أ�h��ClԪ�ء�
��1��x��HԪ�أ�HԪ���γɵ�������ΪH-��H-�Ľṹʾ��ͼΪ![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
��2��z��NԪ�أ�Nԭ�ӵ�������Ϊ7�����Nԭ���к�8�����ӣ���������Ϊ7+8=15������ԭ�ӷ���Ϊ![]() N���ʴ�Ϊ��
N���ʴ�Ϊ��![]() N��
N��
��3��f��AlԪ�أ�e��NaԪ�أ�Na������������Ӧ��ˮ����ΪNaOH��Al��NaOH��Һ��Ӧ����ƫ�����ƺ���������Ӧ�Ļ�ѧ����ʽΪ��2Al+2NaOH+2H2O=2NaAlO2+3H2�����ʴ�Ϊ��2Al+2NaOH+2H2O=2NaAlO2+3H2����
��4��d��OԪ�أ�e��NaԪ�أ��γɵļ�����ΪO2-��Na+�����Ӳ�ṹ��ͬ�����ӣ��˵����Խ�����Ӱ뾶ԽС����r(O2-)��r(Na+)���ʴ�Ϊ��O2- >Na+��
��5��g��SԪ�ء�h��ClԪ�أ�Ԫ�صķǽ�����Խǿ�����������ˮ���������Խǿ���ǽ����ԣ�Cl��S������������Ӧˮ���������HClO4��H2SO4���ʴ�Ϊ��HClO4��
��6��x��HԪ�أ�z��NԪ�أ�d��OԪ�أ���H��N��O����Ԫ�صĻ�����ΪHNO3��NH3��H2O��HNO3��NH3��H2O�����кͷ�Ӧ����NH4NO3��H2O����Ӧ�����ӷ���ʽΪ��H++NH3��H2O=NH4++H2O���ʴ�Ϊ��H++NH3��H2O=NH4++H2O��