��Ŀ����
��12�֣���֪һ��̼ԭ����ͬʱ���������ǻ�ʱ����������ת��: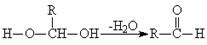
�����ͼʾ�ش�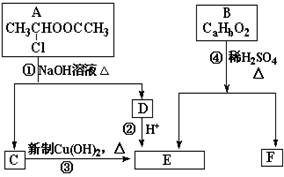
��1��E�к��й����ŵ�������_________���۵ķ�Ӧ������______________��C�����Ƶ�������ͭ����Һ��Ӧ�Ļ�ѧ����ʽΪ ��
��2����֪B����Է�������Ϊ162������ȫȼ�յIJ�����n(CO2)��n(H2O) =" 2" ��1��F�ǵ���оƬ�и߷��ӹ��������Ҫԭ�ϣ�F���������ص㣺���ܸ�FeCl3��Һ������ɫ��Ӧ�����ܷ����Ӿ۷�Ӧ���۱����ϵ�һ�ȴ���ֻ�����֡���F��һ�������·����Ӿ۷�Ӧ�Ļ�ѧ����ʽΪ__ _______________________________________________ _��
��3��������G��F��ͬ���칹�壬�����ڷ����廯����ܷ���������Ӧ��G������ �ֽṹ��д������һ��ͬ���칹��Ľṹ��ʽ ______________ ��
��1���ǻ���������ԭ��Ӧ��CH3CHO+2Cu(OH)2 CH3COOH+Cu2O+2H2O��
CH3COOH+Cu2O+2H2O��
��2��
��3��4�� ��
�� ��
�� ��
�� ������һ�֣���
������һ�֣���
�������������A���������Ƶ�ˮ��Һ����ȡ����Ӧ����C��D��C�ܺ�����������ͭ����������ԭ��Ӧ����C�Ľṹ��ʽΪCH3CHO��DΪ���ᣬ�ṹ��ʽΪCH3COONa��D���ữ����E��E�Ľṹ��ʽΪCH3COOH��B����Է�������Ϊ162������ȫȼ�յIJ�����n��CO2����n ��H2O��=2��1����B��̼��ԭ�Ӹ���֮��Ϊ1��1������a=b���������Է�������֪��a= 162?32/13=10������B�ķ���ʽΪC10H10O2��Bˮ�����������F��F���������ص㣺���ܸ�FeCl3��Һ������ɫ��Ӧ�����ܷ����Ӿ۷�Ӧ���۱����ϵ�һ�ȴ���ֻ�����֣���F�Ľṹ��ʽΪ�� ��
��1��E�Ľṹ��ʽΪCH3COOH��E�й������������Ȼ����ʴ�Ϊ���Ȼ���C�ܺ�����������ͭ����������ԭ��Ӧ����C�Ľṹ��ʽΪCH3CHO��E�Ľṹ��ʽΪCH3COOH��C������������ͭ����Һ����������ԭ��Ӧ����E���÷�Ӧ����ʽΪCH3CHO+2Cu(OH)2CH3COOH+Cu2O+2H2O��
��2��F���������ص㣺���ܸ�FeCl3��Һ������ɫ��Ӧ��˵�����з��ǻ������ܷ����Ӿ۷�Ӧ��˵������C=C�����۱����ϵ�һ�ȴ���ֻ�����֣�����������ȡ������λ�ڶ�λλ�ã�ӦΪ ���ɷ����Ӿ۷�Ӧ������ʽΪ
���ɷ����Ӿ۷�Ӧ������ʽΪ ��
��
��3��������G��F��ͬ���칹�壬���ڷ����廯����ܷ���������Ӧ��G�к���ȩ�������ܵ�ͬ���칹���� ��
�� ��
�� ��
�� ��4�֡�
��4�֡�
���㣺�л�����ƶ�

 ��У����ϵ�д�
��У����ϵ�д�Heck��Ӧ�Ǻϳ�C-C������Ч����֮һ���練Ӧ�٣�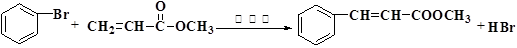
I �� ��
�������������ºϳ�·��ã�
��1�� �������ķ���ʽΪ________��1 mol�������������______mol H2�����ӳɷ�Ӧ��
��2��������IV���ӽṹ���м���д���ɻ�����IV��Ӧ���ɻ�����V�Ļ�ѧ����ʽ________________________________________________________________��
��3���йػ������˵����ȷ����____________
| A��1 mol ���������ȫȼ������5 mol O2 |
| B�����������ʹ���Ը��������Һ��ɫ |
| C���������������ˮ |
D�����������Ӽ�ۺϣ���Ӧ���ɵĸ߾���ṹΪ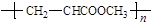 |
��5��
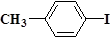 ��
�� Ҳ���Է������Ʒ�Ӧ�ٵķ�Ӧ���л�����Ľṹ��ʽΪ_______________��
Ҳ���Է������Ʒ�Ӧ�ٵķ�Ӧ���л�����Ľṹ��ʽΪ_______________�� ij�о�С���Լױ�Ϊ��Ҫԭ�ϣ���������·�ߺϳ�ҽҩ�м���F��Y��
��֪��
��ش��������⣺
��1�������й�F��˵����ȷ���� ��
| A������ʽ��C7H7NO2Br | B�����γ����� |
| C���ܷ���ȡ����Ӧ�����۷�Ӧ | D��1 mol�� F�����Ժ�2 mol NaOH��Ӧ |
��3�� B��C�Ļ�ѧ����ʽ�� ���ںϳ�F�Ĺ����У�B��C���費��ʡ�ԣ������� ��
��4��D��E��Ӧ������Լ��� ��
��5��д��ͬʱ��������������A��ͬ���칹��Ľṹ��ʽ ��д��3������
�ٱ�����ֻ�����ֲ�ͬ��ѧ��������ԭ�� �ڷ����к���
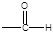
��6����X����ϩΪԭ�Ͽɺϳ�Y������ƺϳ�·�ߣ����Լ����ܼ���ѡ����
ע���ϳ�·�ߵ���д��ʽ��������ʾ������ͼ��
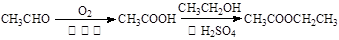

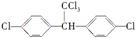 ���л����У������������ ��ԭ�ӹ��档
���л����У������������ ��ԭ�ӹ��档
 ��һ��-Cl�������ܵĽṹ�� �֡������ⲻ���Ƕ�ӳ�칹�壩
��һ��-Cl�������ܵĽṹ�� �֡������ⲻ���Ƕ�ӳ�칹�壩





