��Ŀ����
������أ�K2FeO4�����м�ǿ�������ԣ���һ��������ˮ��������
��1��FeO42-��ˮ��Ӧ�ķ���ʽΪ��4FeO42- + 10H2O  4Fe(OH)3 + 8OH��+ 3O2��
4Fe(OH)3 + 8OH��+ 3O2��
K2FeO4�ڴ���ˮ�Ĺ���������������� �� ��

��2��������K2FeO4���Ƴ�c(FeO42-) ��1.0��10-3 mol��L-1��1.0mmol��L-1�����������������ֱ�����20�桢30�桢40���60��ĺ���ˮԡ�У��ⶨc(FeO42-)�ı仯�������ͼ���⣨1���еķ�ӦΪFeO42-�仯������Ӧ���÷�Ӧ�ġ�H 0��
��3��FeO42-��ˮ��Һ�еĴ�����̬��ͼ����ʾ������˵����ȷ���� ������ĸ����
A��������Һ�������α仯����Ԫ�ض���4�ִ�����̬
B���ı���Һ��pH������Һ��pH��10����pH��4�Ĺ����У�HFeO4���ķֲ�������������С
C����pH��8��������Һ�м�KOH��Һ��������Ӧ�����ӷ���ʽΪ��H2FeO4��OH����HFeO4����H2O
D��pHԼΪ2.5 ʱ����Һ��H3FeO4+��HFeO4�������൱
��4��HFeO4�� H++FeO42-�ĵ���ƽ�ⳣ������ʽΪK=___________________������ֵ�ӽ� ������ĸ����
H++FeO42-�ĵ���ƽ�ⳣ������ʽΪK=___________________������ֵ�ӽ� ������ĸ����
A��10-2.5 B��10-6 C��10-7 D��10-10
��5��25��ʱ��CaFeO4��Ksp = 4.536��10-9����Ҫʹ100mL��1.0��10-3 mol��L-1��K2FeO4��Һ�е�c(FeO42- )��ȫ����������������Ҫ�����Ca(OH)2�����ʵ���Ϊ mol��
��ȫ��������Һ�в�����c(FeO42- )Ϊ______________��
��1��ɱ���������������ʣ�2��>��3��BD��4��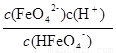 C��5��4.536��10-5��1��10-5mol/L
C��5��4.536��10-5��1��10-5mol/L
��������
�����������1��FeO42-����ǿ�����ԣ�������ɱ����FeO42-��ˮ���ɵ�Fe(OH)3����������ˮ�е������ʹˮ�徻������2����ͼI���Կ������¶�Խ�ߣ���Һ��c(FeO42-)ԽС��˵�������¶ȣ�ƽ�������ƶ�����������Ӧ�����ȷ�Ӧ����3����ͼ��֪��pHС��1.5ʱ����Һ��ֻ��H3FeO4+��H2FeO4��A����pH��10����4ʱ��HFeO4���ķֲ�������������С��B��ȷ����pH=8�ĸ���Һ�м�KOH��Һ��pH�������ӷ���ʽΪHFeO4-+OH-=FeO42-+H2O��C����pHԼΪ2.5 ʱ�� H3FeO4+��HFeO4���ֲ�������ȣ�D��ȷ����4��HFeO4�� H++FeO42-�ĵ���ƽ�ⳣ��K=
H++FeO42-�ĵ���ƽ�ⳣ��K=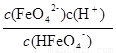 ����pH�ӽ�7ʱ��c(FeO42-)=c(HFeO4-)��K��10-7����5��Ҫ�γ�CaFeO4����������c(Ca2+)��c(FeO42-)>Ksp(CaFeO4)��
����pH�ӽ�7ʱ��c(FeO42-)=c(HFeO4-)��K��10-7����5��Ҫ�γ�CaFeO4����������c(Ca2+)��c(FeO42-)>Ksp(CaFeO4)��
���㣺��ɳ����ԭ�� �ܶȻ����� ����ƽ�ⳣ�� ͼ��ķ���
��������ɳ����ԭ������������ƽ����ϵ��������Һ��ϣ��γɳ�����������Q>Ksp��

 ��һ������Ԫͬ�����ؾ�ϵ�д�
��һ������Ԫͬ�����ؾ�ϵ�д�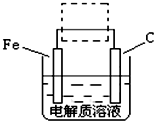 ��FeSO4?7H2O��һ��ˮ����ԭ��Ϊ
��FeSO4?7H2O��һ��ˮ����ԭ��Ϊ 3Zn��OH��2+2Fe��OH��3+4KOH
3Zn��OH��2+2Fe��OH��3+4KOH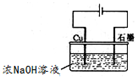 ��Һ��pHΪ
��Һ��pHΪ