��Ŀ����
����ͭ���仯����������������й㷺Ӧ�ã���ش��������⣺
��1��������FeS2������SΪ-1�ۣ������������ұ����������Ҫԭ�ϣ�����һ����ӦΪ3FeS2+8O2
6SO2+Fe3O4����������Ϊ
��2�����������ƣ�������Ҳ��������ˮ����ij����ˮ�����������̷�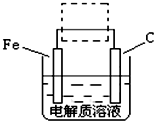 ��FeSO4?7H2O��һ��ˮ����ԭ��Ϊ
��FeSO4?7H2O��һ��ˮ����ԭ��Ϊ
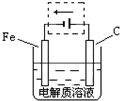
��3���ٸ����ĵ绯��ʴ��ʾ��ͼ���ң�����ͼ������ ���ɳ�Ϊ�����绯ѧ�����ļ�ʾ��ͼ��������ͼ���߿��������ģ����ü�ͷ���������������
��д����ǰ�ĸ���������ʴʯī�缫�ĵ缫��Ӧʽ
��4��������أ�K2FeO4����������ˮ����Ҳ���������������أ����������һ�����Ϳɳ���أ�����ͨ���ܵ����ȣ��õ���ܳ�ʱ�䱣���ȶ��ķŵ��ѹ��������ص��ܷ�ӦΪ
3Zn+2K2FeO4+8H2O 3Zn��OH��2+2Fe��OH��3+4KOH
3Zn��OH��2+2Fe��OH��3+4KOH
�õ�طŵ�ʱ������ӦʽΪ
�øõ�ص��100mL 1mol?L-1��AgNO3��Һ������·��ͨ��0.1mol ����ʱ�������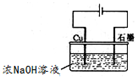 ��Һ��pHΪ
��Һ��pHΪ
��5��������ͭ��һ�ְ뵼����ϣ������������������IJ��ϣ����������ڹ���������ˮ�ֽ�Ĵ�����ũ�����ɱ������
����ͼװ�ÿ��Ƶ�������ͭ����д�������ĵ缫��Ӧʽ
��1��������FeS2������SΪ-1�ۣ������������ұ����������Ҫԭ�ϣ�����һ����ӦΪ3FeS2+8O2
| ||
SO2��Fe3O4
SO2��Fe3O4
������3mol FeS2�μӷ�Ӧ��ת�Ƶ��ӵ����ʵ���Ϊ32mol
32mol
����2�����������ƣ�������Ҳ��������ˮ����ij����ˮ�����������̷�
 ��FeSO4?7H2O��һ��ˮ����ԭ��Ϊ
��FeSO4?7H2O��һ��ˮ����ԭ��ΪCl2+2Fe2+=2Fe3++2Cl-
Fe3++3H2O?Fe��OH��3�����壩+3H+
Fe3++3H2O?Fe��OH��3�����壩+3H+
Cl2+2Fe2+=2Fe3++2Cl-
Fe3++3H2O?Fe��OH��3�����壩+3H+
�������ӷ���ʽ��ʾ����Fe3++3H2O?Fe��OH��3�����壩+3H+
��3���ٸ����ĵ绯��ʴ��ʾ��ͼ���ң�����ͼ������ ���ɳ�Ϊ�����绯ѧ�����ļ�ʾ��ͼ��������ͼ���߿��������ģ����ü�ͷ���������������
��д����ǰ�ĸ���������ʴʯī�缫�ĵ缫��Ӧʽ
O2+2H2O+4e-=4OH-��
O2+2H2O+4e-=4OH-��
����4��������أ�K2FeO4����������ˮ����Ҳ���������������أ����������һ�����Ϳɳ���أ�����ͨ���ܵ����ȣ��õ���ܳ�ʱ�䱣���ȶ��ķŵ��ѹ��������ص��ܷ�ӦΪ
3Zn+2K2FeO4+8H2O
 3Zn��OH��2+2Fe��OH��3+4KOH
3Zn��OH��2+2Fe��OH��3+4KOH�õ�طŵ�ʱ������ӦʽΪ
2FeO42-+8H2O+6e-=2Fe��OH��3+10OH-
2FeO42-+8H2O+6e-=2Fe��OH��3+10OH-
���øõ�ص��100mL 1mol?L-1��AgNO3��Һ������·��ͨ��0.1mol ����ʱ�������
 ��Һ��pHΪ
��Һ��pHΪ0
0
����Һ����仯���Բ��ƣ�����5��������ͭ��һ�ְ뵼����ϣ������������������IJ��ϣ����������ڹ���������ˮ�ֽ�Ĵ�����ũ�����ɱ������
����ͼװ�ÿ��Ƶ�������ͭ����д�������ĵ缫��Ӧʽ
2Cu-2e-+2OH-=Cu2O+H2O
2Cu-2e-+2OH-=Cu2O+H2O
����������1�����ݻ��ϼ��ж��������ͻ�ԭ�������ݵ��ӵ�ʧ���ó�SO2��������Ĺ�ϵ��
��2�����������ܽ�Fe2+������Fe3+���Լ�Fe3+ˮ������Fe��OH��3������Ծ�ˮ��
��3���ٸ��ݽ����ķ����������ڸ���������ʴд�������ĵ缫��Ӧ��
��4����д�������ĵ缫��Ӧ�������ܷ�Ӧ-�����ĵ缫��Ӧ=�����ĵ缫��Ӧ�����ݵ缫��Ӧ�����㣻
��5������ͭ�������Լ���Ϣ�����
��2�����������ܽ�Fe2+������Fe3+���Լ�Fe3+ˮ������Fe��OH��3������Ծ�ˮ��
��3���ٸ��ݽ����ķ����������ڸ���������ʴд�������ĵ缫��Ӧ��
��4����д�������ĵ缫��Ӧ�������ܷ�Ӧ-�����ĵ缫��Ӧ=�����ĵ缫��Ӧ�����ݵ缫��Ӧ�����㣻
��5������ͭ�������Լ���Ϣ�����
����⣺��1����Ӧ3FeS2+8O2
Fe3O4+6SO2��ǰ���Ԫ�ػ��ϼ۵ı仯������£�
Fe��+2��+3�����ϼ����ߣ�S��-1��+4�����ϼ����ߣ�
O��0��-2�����ϼ۽��ͣ�
��ˣ��ڷ�Ӧ��FeS2��ԭ����O2����������Fe2O3������������Ҳ�ǻ�ԭ���SO2������������Ҳ�ǻ�ԭ�����������Ӧ�й�ת��32�����ӣ���3molFeS2�μӷ�Ӧ��ת�Ƶ��ӵ����ʵ���Ϊ32mol���ʴ�Ϊ��SO2��Fe3O4��32mol��
��2���������ܽ�Fe2+������Fe3+���Լ�Fe3+ˮ������Fe��OH��3������Ծ�ˮ���ʴ�Ϊ��Cl2+2Fe2+=2Fe3++2Cl-
Fe3++3H2O Fe��OH��3+3H+��
Fe��OH��3+3H+��
��3���ٸ�������������������������֪����Ҫ�����Ľ�������������ͼ����ʾ��
��������ʴ�������ĵ缫��ӦΪ��O2+2H2O+4e-=4OH-���ʴ�Ϊ��O2+2H2O+4e-=4OH-��
��4�������ĵ缫��Ӧ��3Zn+6OH-=3Zn��OH��2�������ĵ缫��Ӧ��2FeO42-+8H2O+6e-=2Fe��OH��3+10OH-��
�ʴ�Ϊ��2FeO42-+8H2O+6e-=2Fe��OH��3+10OH-��
�����ĵ缫��Ӧ��40H--4e-=2H20+O2��
0.1mol 0.1mol
���Բ�����H+�����ʵ���Ϊ 0.1mol�������ʵ���Ũ��Ϊ1mol/L������PH=0���ʴ�Ϊ��0��
��5��ͭ��������������Cu2O�������ĵ缫��Ӧʽ��2Cu-2e-+2OH-=Cu2O+H2O���ʴ�Ϊ��2Cu-2e-+2OH-=Cu2O+H2O��
| ||
Fe��+2��+3�����ϼ����ߣ�S��-1��+4�����ϼ����ߣ�
O��0��-2�����ϼ۽��ͣ�
��ˣ��ڷ�Ӧ��FeS2��ԭ����O2����������Fe2O3������������Ҳ�ǻ�ԭ���SO2������������Ҳ�ǻ�ԭ�����������Ӧ�й�ת��32�����ӣ���3molFeS2�μӷ�Ӧ��ת�Ƶ��ӵ����ʵ���Ϊ32mol���ʴ�Ϊ��SO2��Fe3O4��32mol��
��2���������ܽ�Fe2+������Fe3+���Լ�Fe3+ˮ������Fe��OH��3������Ծ�ˮ���ʴ�Ϊ��Cl2+2Fe2+=2Fe3++2Cl-
Fe3++3H2O
 Fe��OH��3+3H+��
Fe��OH��3+3H+����3���ٸ�������������������������֪����Ҫ�����Ľ�������������ͼ����ʾ��

��������ʴ�������ĵ缫��ӦΪ��O2+2H2O+4e-=4OH-���ʴ�Ϊ��O2+2H2O+4e-=4OH-��
��4�������ĵ缫��Ӧ��3Zn+6OH-=3Zn��OH��2�������ĵ缫��Ӧ��2FeO42-+8H2O+6e-=2Fe��OH��3+10OH-��
�ʴ�Ϊ��2FeO42-+8H2O+6e-=2Fe��OH��3+10OH-��
�����ĵ缫��Ӧ��40H--4e-=2H20+O2��
0.1mol 0.1mol
���Բ�����H+�����ʵ���Ϊ 0.1mol�������ʵ���Ũ��Ϊ1mol/L������PH=0���ʴ�Ϊ��0��
��5��ͭ��������������Cu2O�������ĵ缫��Ӧʽ��2Cu-2e-+2OH-=Cu2O+H2O���ʴ�Ϊ��2Cu-2e-+2OH-=Cu2O+H2O��
���������⿼���֪ʶ��϶࣬��һ�����Ѷȣ�ע�����֪ʶ�Ļ��ۣ�

��ϰ��ϵ�д�
 ��������ϵ�д�
��������ϵ�д� ���ɶ���ܲ��¿�ֱͨ�߿�ϵ�д�
���ɶ���ܲ��¿�ֱͨ�߿�ϵ�д�
�����Ŀ
