��Ŀ����
����Ŀ����������ƾ��壨Na2S2O3��5H2O��M=248 g��mol1����������Ӱ������ԭ�����ش��������⣺
��1����֪��Ksp(BaSO4)=1.1��1010��Ksp(BaS2O3)=4.1��105����������������г�������������ʣ�ѡ�������Լ����ʵ�鷽�����м��飺
�Լ���ϡ���ᡢϡH2SO4��BaCl2��Һ��Na2CO3��Һ��H2O2��Һ
��������
ʵ�鲽�� | ���� |
��ȡ������Ʒ�������������ˮ | �ڹ�����ȫ�ܽ����ɫ������Һ |
�ۼ������ϡ���� | ��___________���д̼���������� |
�ݾ��ã�������ȡ�ϲ���Һ������BaCl2��Һ | ��_______________________________ |
��2������K2Cr2O7����Һ�����ⶨ��������ƵĴ��ȡ��ⶨ�������£�
����Һ���ƣ���ȡ1.2000 gij��������ƾ�����Ʒ��������в���ȴ������ˮ���ձ����ܽ⣬��ȫ�ܽ��ȫ��ת����100 mL��_________�У�������ˮ�����ߡ�
�ڵζ�����___________________ȡ0.00950 mol��L1��K2Cr2O7����Һ20.00 mL�������ữ��������KI��������Ӧ�� Cr2O72��+6I+14H+=3I2+2Cr3++7H2O��Ȼ���������������Ʒ��Һ�ζ���������ɫ��������Ӧ��I2+2S2O32��=S4O62��+2I�����������Һ��Ϊָʾ���������ζ�������Һ_____________________����Ϊ�յ㡣ƽ�еζ�3�Σ���Ʒ��Һ��ƽ������Ϊ24.80 mL������Ʒ����Ϊ_________%������1λС������
���𰸡������ɫ��ɫ�������� �а�ɫ�������� ����ƿ ��ʽ�ζ��� ��ɫ��Ϊ������ɫ���Ұ���Ӳ��仯 95.0
��������
��1��Na2S2O3�����ᷴӦ�������ʡ����������ˮ�������ڼ������������ʱ����Ҫ�ȼ������ὫS2O32-��ȥ���ټ����Ȼ�����Һ���м��飻
��2������Һ������Ҫ���㡢�������ܽⲢ��ȴ��ת�ơ�ϴ��ת�ơ����ݡ�ҡ�ȵȲ��裬������ƹ���ѡ����Ҫ��������
���������������Ʒ��Һ�ζ���������ɫ��������Ӧ��I2+2S2O32-=S4O62-+2I-�����������Һ��Ϊָʾ������Һ����ɫ�������ζ�����Ӧ�յ㣬�ⵥ�ʷ�Ӧ����Һ��ɫ��ȥ�Ұ���Ӳ��ָ�����ϻ�ѧ����ʽ������ϵ���㣬Cr2O72-+6I-+14H+=3I2+2Cr3++7H2O��I2+2S2O32-=S4O62-+2I-���õ�Cr2O72-��3I2��6S2O32-���ݴ˼��㡣
(1)ȡ������Ʒ���������������ˮ��������ȫ�ܽ����ɫ������Һ���������Һ��������ϡ���ᣬ�����ɫ��ɫ�����������ɣ������Ĵ̼�������Ϊ�����������壬���ã�ȡ�ϲ���Һ�����еμӼ����Ȼ�����Һ���а�ɫ��������֤������������ӣ�
�ʴ�Ϊ�������ɫ��ɫ�������ɣ��а�ɫ�������ɣ�
(2)����Һ������Ҫ���㡢�������ܽⲢ��ȴ��ת�ơ�ϴ��ת�ơ����ݡ�ҡ�ȵȲ��裬������ƹ���ѡ����Ҫ����������ȡ1.2000gij��������ƾ�����Ʒ��������в���ȴ������ˮ���ձ����ܽ⣬��ȫ�ܽ��ȫ��ת����100mL������ƿ�У�������ˮ����Һ����ʹ���̶�����ƽ��
�ʴ�Ϊ������ƿ��
��K2Cr2O7��Һ���������ԣ���������ʽ�ζ���ȡ0.00950molL1��K2Cr2O7����Һ20.00mL�������ữ��������KI��������Ӧ��Cr2O72+6I+14H+=3I2+2Cr3++7H2O��Ȼ���������������Ʒ��Һ�ζ���������ɫ��������Ӧ��I2+2S2O32=S4O62+2I�����������Һ��Ϊָʾ������Һ����ɫ�������ζ�������Ӧ�յ㣬�ⵥ�ʷ�Ӧ����Һ��ɫ��ȥ�Ұ���Ӳ��ָ���˵����Ӧ����ζ��յ㣬ƽ�еζ�3�Σ���Ʒ��Һ��ƽ������Ϊ24.80mL����ϻ�ѧ����ʽ������ϵ���㣬Cr2O72+6I+14H+=3I2+2Cr3++7H2O��I2+2S2O32=S4O62+2I���õ�Cr2O723I26S2O32����n��Na2S2O3��=6n��K2Cr2O7��=0.0095mol/L��0.02L��6=0.00114mol�����Ƶ�100mL��Һ��n(S2O32)=0.00114 mol��![]() =0.0046mol������Ʒ����=
=0.0046mol������Ʒ����=![]() ��100%=95.0%��
��100%=95.0%��
�ʴ�Ϊ����ʽ�ζ��ܣ���ɫ��Ϊ������ɫ���Ұ���Ӳ��仯��95.0��

����Ŀ�����û�ѧԭ�����ԶԹ����ŷŵķ�ˮ�������Ƚ�����Ч��������������ij�������Ƹ﹤ҵ������Cr(III)�Ĵ��������������¡�

���������ȡҺ�еĽ���������Ҫ��Cr3+�������Fe3+��Al3+��Ca2+��Mg2+��
��1�����ʱ��Ϊ����߽�ȡ�ʿɲ�ȡ�Ĵ�ʩ��______________![]() �������
�������![]() ��
��
��2��H2O2�������ǽ���Һ���е�Cr3+ת��ΪCr2O72-��д���˷�Ӧ�����ӷ���ʽ��_____________��
��3�������£�����������������������ʽ����ʱ��Һ��pH���£�
������ | Fe3+ | Mg2+ | Al3+ | Cr3+ |
��ʼ����ʱ��pH | 2.7 |
|
|
|
������ȫʱ��pH | 3.7 | 11.1 | 8 | 9(>9�ܽ�) |
����NaOH��Һʹ��Һ�ʼ��ԣ�Cr2O72-ת��ΪCrO42-����Һ������������Ҫ��________������Һ��pH���ܳ���8����������_______________��
��4��д��������������SO2���л�ԭʱ������Ӧ�Ļ�ѧ����ʽ�� ____________��
��5���ζ����ⶨ�����Ʒ��NaHCO3�����ķ����ǣ�ȷ��ȡ������ƷWg��������ƿ�м�����ˮ�ܽ⣬��1~2�η�ָ̪ʾ������cmol/L��HCl��Һ�ζ�����Һ�ɺ�ɫ��Ϊ��ɫ![]() ָʾ
ָʾ![]() ��Ӧ���յ�
��Ӧ���յ�![]() ������HCl��Һ���ΪV1mL���ټ�1~2�μ���ָʾ����������HCl��Һ�ζ�����Һ�ɻ�ɫ��Ϊ��ɫ������HCI��Һ�����ΪV2mL������Ʒ��NaHCO3��������Ϊ____________��
������HCl��Һ���ΪV1mL���ټ�1~2�μ���ָʾ����������HCl��Һ�ζ�����Һ�ɻ�ɫ��Ϊ��ɫ������HCI��Һ�����ΪV2mL������Ʒ��NaHCO3��������Ϊ____________��
����Ŀ�����ȷ�Ӧ������һ����Ҫ���ʣ���������;ʮ�ֹ㷺�� �����ȷ�Ӧ��������������������������Ӧ�ų��������ȣ�������ҫ�۵Ĺ�â����ֽ©�����²����մ���������������ɳ�С�����֪��Al��Al2O3��Fe��Fe2O3���۵㡢�е��������£�
���� | Al | Al2O3 | Fe | Fe2O3 |
�۵�/�� | 660 | 2 054 | 1 535 | 1 462 |
�е�/�� | 2 467 | 2 980 | 2 750 | �� |
I.��1��ijͬѧ�Ʋ⣬���ȷ�Ӧ���õ���������Ӧ�������Ͻ������ǣ��÷�Ӧ�ų�������ʹ���ۻ����������۵�����ͣ���ʱҺ̬���������ۺ��γ������Ͻ�����Ϊ���Ľ����Ƿ������________(���������������)��
��2�����һ����ʵ�鷽����֤���������õĿ�״�������к��н���������ʵ�������Լ���________����Ӧ�����ӷ���ʽΪ______________________________________��
��3��ʵ�����ܽ��������������Լ��������˵��Լ���________������ţ���
A��Ũ���� B��ϡ���ᡡ������C��ϡ���� D������������Һ
��.ʵ���о����֣����ᷢ��������ԭ��Ӧʱ�������Ũ��Խϡ����Ӧ��ԭ�����е�Ԫ�صĻ��ϼ�Խ�͡�ijͬѧȡһ������������������һ������ϡ�������ַ�Ӧ����Ӧ������������ų����ڷ�Ӧ���������Һ�У���μ���6mol��L��1������������Һ����������������Һ�����(mL)������ij��������ʵ���(mol)�Ĺ�ϵ��ͼ��ʾ���Իش��������⣺
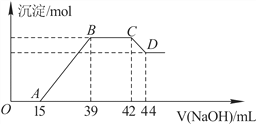
��1�������⣬��д���������������ϡ�����ᷴӦ�����ӷ���ʽ��____________________
��2��ͼ��OA��û�г������ɣ��˽η�����Ӧ�����ӷ���ʽΪ__________________��
��3����BC�Σ����������ʵ���û�б仯����˽η�����Ӧ�����ӷ���ʽΪ_____________��
��4������������Ԫ�ص����ʵ���Ϊ________mol��
��5��B���Ӧ�ij��������ʵ���Ϊ______mol��A���Ӧ������������Һ�����Ϊ________mL��
����Ŀ����֪HNO2�ڵ����½��ȶ����������������л�ԭ�ԣ������������ԭ��������ҺpH�Ĺ�ϵ���±��������ԣ�![]() �������й�˵����������
�������й�˵����������![]()
![]()
pH��Χ |
|
|
���� |
| NO�� |
A.���������£�NaNO2��NaClO��Ӧ�����ӷ���ʽΪ![]()
B.�����NaNO2��Һ��ͨ��H2S���壬�е���ɫ��������
C.����ʱ���������Լ���������HNO2��Һ��Na2CO3��Һ
D.�����NaNO2��Һ��ͨ��SO2�ɵõ�HNO2