��Ŀ����
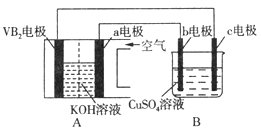
����Ŀ��һ�������£��������ʵ������ת��:
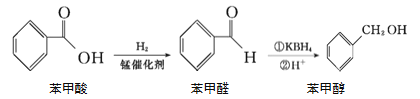
��1��Mn2+��̬��������Ų�ʽΪ________��
��2��B��C��O��K �ĵ�һ��������С�����˳����________��
��3��1mol��������Ӻ�����������ĿΪ________�����״�������Oԭ�ӵĹ���ӻ�����Ϊ____��
��4��KBH4������BH4-���ӿռ乹��Ϊ_____,д��һ����BH4-��Ϊ�ȵ�����������ӵĻ�ѧʽ:_________��
��5�������ᡢ����ȩ�����״����������зе���͵���____��ԭ����_____________��
���𰸡� ls22s22p63s23p63d5����[Ar]3d5�� K��B��C��O 15 mol sp3 ���������� NH4+ ����ȩ ����ȩ���Ӽ䲻��������������������ʷ��Ӽ�������
��������(1)����25��Ԫ�أ�Mn2+��̬��������Ų�ʽΪls22s22p63s23p63d5���ʴ�Ϊ��ls22s22p63s23p63d5��
(2)ͬ���ڣ���һ��Ԫ�صĵ�һ��������С�����һ��Ԫ�صĵ�һ���������������ִ�������������ı仯���ơ�ͬ��Ԫ�أ��������µ�һ��������С������Χ������������ȵĹ�����γ�ȫ��(p0��d0��f0)������(p3��d5��f7)��ȫ��(p6��d10��f14)�ṹʱ��ԭ�ӵ������ϵͣ�Ԫ�صĵ�һ�����ܽϴ�B��C��O��K�ĵ�һ��������С�����˳��ΪK��B��C��O���ʴ�Ϊ��K��B��C��O��
(3)1mol����������к���5molC-H��7molC-C��1molC=O��1molC-O��1molO-H������������15mol�����״�������Oԭ������2��ԭ�ӣ���2���µ��Ӷԣ�����sp3�ӻ����ʴ�Ϊ��15 mol��sp3��
(4)KBH4������BH4-������Bԭ�Ӳ���sp3�ӻ����ռ乹��Ϊ���������Σ���BH4-��Ϊ�ȵ������������ΪNH4+���ʴ�Ϊ�����������Σ�NH4+��
(5)����ȩ���Ӽ䲻��������������������ʷ��Ӽ������������Ӽ�����ܹ�ʹ���ʵķе����ߣ����ᡢ����ȩ�����״����������зе���͵��DZ���ȩ���ʴ�Ϊ������ȩ������ȩ���Ӽ䲻��������������������ʷ��Ӽ���������