��Ŀ����
����Ŀ��һ���¶��£���һ�������ı������ˮϡ�����У���Һ�ĵ��������仯��ͼ��ʾ���й�˵����ȷ����
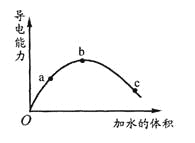
A. a��b��c������Һ��pH��a��b��c
B. ��ʹC���c(CH3COO-)������Һ��pHҲ���ɼ���������CH3COONa����
C. a��b��c������Һ��1mol/L����������Һ�кͣ���������������Һ�����a��b��c
D. ��ʪ���pH��ֽ����c����Һ��pHֵ����ʵ��pHֵƫС
���𰸡�B
��������
A. ��Һ�ĵ�������������Ũ�ȼƼ�������������йأ��ڴ�����Һ�У���Ҫ���ڵ�������H+��CH3COO-����c(H+)=c(CH3COO-)�����ڵ�������b��a��c������c(H+)��b��a��c������Һ��pH��c��a��b��A����
B. �ڴ�����Һ�У�CH3COOH![]() H++CH3COO-������������CH3COONa��c(CH3COO-)����ƽ�������ƶ���c(H+)��С��pH����B��ȷ��
H++CH3COO-������������CH3COONa��c(CH3COO-)����ƽ�������ƶ���c(H+)��С��pH����B��ȷ��
C. �÷�ӦΪCH3COOH+NaOH=CH3COONa+H2O�������������CH3COOH������ͬ��������������������ͬNaOH��Һ�����Ҳ��ͬ��C����
D. ��ʪ���pH��ֽ����c����Һ��pHֵ���൱�ڽ���Һ������ϡ�ͣ���c(H+)ƫС��pHƫ��D����
�ʺ���ѡ��ΪB��

����Ŀ�����𡢵����ס�ͭ��п�Ļ�������������Ҫ��;���ش��������⣺
��1����̬Bԭ�ӵ���ռ������ܼ��ĵ���������ͼΪ____����̬Cu+�ĺ�������Ų�ʽΪ___��
��2��������(CH3)3N������ˮ��ԭ����______��
��3�������ᣨH3PO3������Ԫ�ص�һ�ֺ����ᣬ��NaOH��Ӧֻ����NaH2PO3��Na2HPO3�����Σ���H3PO3���ӵĽṹʽΪ____��
��4��Zn2+����CN�����������꣨![]() �����γ��ȶ�����
�����γ��ȶ�����
��CN�� �ĽṹʽΪ_____��
��ÿ��������������У���ȡsp2�ӻ���ԭ����__����
��5��±��п���۵������ʾ��
ZnF2 | ZnCl2 | ZnBr2 | ZnI2 | |
�۵�/�� | 872 | 275 | 394 | 446 |
ZnF2���۵�Զ������������±��п����ԭ��Ϊ_____��
��6��������һ����ĥͿ�ϣ��������������ı��汣���㡣�����徧����ͼ1��ʾ��
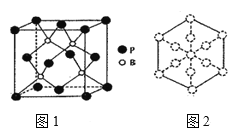
������������Խ��߷����ͶӰ��ͼ2�����ڴ���Ͻ���ʾBԭ�ӵ�ԲȦͿ��____��
����֪��������ܶ�Ϊ�� g/cm3�������ӵ�����ΪNA����B��P����Ϊ___pm���г�����ʽ���ɣ���

