��Ŀ����
��14�֣���2L����ѹ�Ƶĺ����ܱ�������ͨ��2molX��g����1molY��g����������Ӧ��
2X��g��+Y��g�� 2Z��g������H��0���ﵽƽ��ʱ���������ʵ�����Ϊԭ����0.85������ش��������⡣
2Z��g������H��0���ﵽƽ��ʱ���������ʵ�����Ϊԭ����0.85������ش��������⡣
��1��ƽ��ʱZ�����ʵ���Ϊ___________������Ӧ����5min�ﵽƽ�⣬��Y��ƽ����Ӧ����Ϊ____________��
��2����ͬ�����½��з�Ӧ����t1ʱ�̣�ֻ�ı�����ijһ��������������ͼ����ͼ�ס�
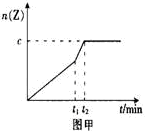
A������ B������ C����ѹ D����ѹ E���Ӵ���
��c=0.90mol��t1ʱ�̸ı��������_________����ѡ���ţ���ͬ����t2_______5min (���������������=����ͬ����
��3���������������䣬ԭ����Ϊ��ѹ�������ﵽƽ���Z�����ʵ���______0.9mol (���������������=������
��4���������ʵ�����Ϊ3.00mol����X��Y�����5L�����з�����Ӧ���ڷ�Ӧ������Z�����ʵ����������¶ȱ仯��ͼ�ҡ�
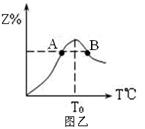
��A��B����Z��������Ӧ���ʵĴ�С��ϵ��_________��
���¶�T��T0ʱ��Z%�������ԭ����______________��
2X��g��+Y��g��
 2Z��g������H��0���ﵽƽ��ʱ���������ʵ�����Ϊԭ����0.85������ش��������⡣
2Z��g������H��0���ﵽƽ��ʱ���������ʵ�����Ϊԭ����0.85������ش��������⡣��1��ƽ��ʱZ�����ʵ���Ϊ___________������Ӧ����5min�ﵽƽ�⣬��Y��ƽ����Ӧ����Ϊ____________��
��2����ͬ�����½��з�Ӧ����t1ʱ�̣�ֻ�ı�����ijһ��������������ͼ����ͼ�ס�

A������ B������ C����ѹ D����ѹ E���Ӵ���
��c=0.90mol��t1ʱ�̸ı��������_________����ѡ���ţ���ͬ����t2_______5min (���������������=����ͬ����
��3���������������䣬ԭ����Ϊ��ѹ�������ﵽƽ���Z�����ʵ���______0.9mol (���������������=������
��4���������ʵ�����Ϊ3.00mol����X��Y�����5L�����з�����Ӧ���ڷ�Ӧ������Z�����ʵ����������¶ȱ仯��ͼ�ҡ�

��A��B����Z��������Ӧ���ʵĴ�С��ϵ��_________��
���¶�T��T0ʱ��Z%�������ԭ����______________��
��1��0.9mol ��2�֣� 0.045 mol?L-1?min-1��2�֣�
��2��E��2�֣��� �� ��2�֣�
��3������2�֣�
��4����B��A����2�֣�
�ڷ�Ӧδ�ﵽƽ�⣬��Ӧ����������Ӧ������У���Z%��������2�֣�
��2��E��2�֣��� �� ��2�֣�
��3������2�֣�
��4����B��A����2�֣�
�ڷ�Ӧδ�ﵽƽ�⣬��Ӧ����������Ӧ������У���Z%��������2�֣�
���������
��1�� 2X��g��+Y��g��
 2Z��g��
2Z��g��n(ʼ) 2mol 1mol 0 mol
n(��) 2a mol a mol 2a mol
n(��) 2-2a mol 1-a mol 2a mol
3��0.85="2-2a+1-a+2a" ������a="0.45" ��ƽ��ʱZ�����ʵ���=2a=0.9mol��Y�ķ�Ӧ����=0.45/(5��2)= 0.045 mol?L-1?min-1��
��2�� ֻ�Ǽ����˷�Ӧ�ﵽƽ��ģ�����Ӧ�Ǽ����˴�����ʱ��ӦС��ԭ����5���ӡ�
��3�� ��ѹ��ԭ��Ӧ���������У����������С�������������Ҳ�ڼ�С������Ϊ���ݣ����������С��ѹǿ��С�������ڷ�Ӧ������Ӧ�����Ժ�ѹ��Z�����ʵ�������0.9mol��
��4�� ��B����¶ȸ���A�㣬�¶����ߣ���Ӧ��������
��Z��δ�ﵽƽ��״̬������Ӧ���ʴ����淴Ӧ���ʣ�ƽ������Ӧ������Z�İٷֺ���������ߡ�
��������������ѶȲ������ڳ�����㡣�����ʶͼ������Ҫץס�¶ȶԷ�Ӧ���ʵ�Ӱ�졣

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
 CO2 (g)������˵���д������
CO2 (g)������˵���д������ 2SO3(g)��SO2��ת�������¶ȵı仯���±���ʾ��
2SO3(g)��SO2��ת�������¶ȵı仯���±���ʾ��
 .�۴��¶��µ�ƽ�ⳣ��Ϊ �����÷�����ʾ��.
.�۴��¶��µ�ƽ�ⳣ��Ϊ �����÷�����ʾ��. CO2(g) + H2(g)�ﵽƽ�⣬�������������������£����д�ʩ����ʹ����Ӧ���ʼӿ���� �� ��
CO2(g) + H2(g)�ﵽƽ�⣬�������������������£����д�ʩ����ʹ����Ӧ���ʼӿ���� �� �� cC(g) ����������:v����K1��c(A)��a?��c(B)��b,v����K2?��c(C)��c��K1��K2���¶�һ��ʱΪ��������������������ʱ����ѹǿ����һ����v��ӦΪԭ����( )
cC(g) ����������:v����K1��c(A)��a?��c(B)��b,v����K2?��c(C)��c��K1��K2���¶�һ��ʱΪ��������������������ʱ����ѹǿ����һ����v��ӦΪԭ����( ) 2NO�����������ܼӿ��䷴Ӧ���ʵ���
2NO�����������ܼӿ��䷴Ӧ���ʵ���