��Ŀ����
��13�֣���ʢ������ͭˮ��Һ���Թ�����백ˮ�������γ�����������Ӱ�ˮ���������ܽ⣬�õ�����ɫ������Һ�������� ���Խ�С���ܼ�(���Ҵ�)������������ɫ�ľ��塣
���Խ�С���ܼ�(���Ҵ�)������������ɫ�ľ��塣
(��)�ڻ�ѧʵ��Ϳ�ѧ�о��У�ˮ��һ����õ��ܼ���ˮ������֮Դ���������ǵ�����������ء�
��1�� ���
д���� ���ӻ�Ϊ�ȵ�������� ����1�֣���
���ӻ�Ϊ�ȵ�������� ����1�֣���
��2��ˮ�������ض����������õ�һ�� ���γ�ˮ�������ӣ�
���γ�ˮ�������ӣ� �������ж��������̵�������
�������ж��������̵������� �������� ��
�������� ��
��3�� �����ڵ�O-H�������Ӽ�ķ��»����������ǿ��������Ϊ_____________
�����ڵ�O-H�������Ӽ�ķ��»����������ǿ��������Ϊ_____________
(��)�������������Ʋ�����Һ����Ҫԭ�ϣ�������Һ��һ�ֱ�����ɱ�������㷺Ӧ������ľ�������ͻ����ϡ�
(4)д��ͭԭ�Ӽ۵��Ӳ�ĵ����Ų�ʽ ����ͭͬһ���ڵĸ���Ԫ�صĻ�̬ԭ����������������ͭԭ����ͬ��Ԫ���� (��Ԫ�ط���)��
(5) ��ʢ������ͭˮ��Һ���Թ�����백ˮ�������γ�����������Ӱ�ˮ���������ܽ⣬�õ�����ɫ������Һ�����Ϲ����з��������ӷ�Ӧ����ʽΪ��
_______________________________��_______________________________��ʵ��ʱ�γɵ�����ɫ��Һ�е��������ڴ��ڵ�ȫ����ѧ�������� ��
(6)ʵ������м���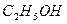 ��ɹ۲쵽��������ɫ
��ɹ۲쵽��������ɫ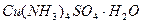 ���塣ʵ��������
���塣ʵ��������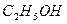 ��������__________________________________________________
��������__________________________________________________
 ���Խ�С���ܼ�(���Ҵ�)������������ɫ�ľ��塣
���Խ�С���ܼ�(���Ҵ�)������������ɫ�ľ��塣(��)�ڻ�ѧʵ��Ϳ�ѧ�о��У�ˮ��һ����õ��ܼ���ˮ������֮Դ���������ǵ�����������ء�
��1��
 ���
д���� ���ӻ�Ϊ�ȵ�������� ����1�֣���
���ӻ�Ϊ�ȵ�������� ����1�֣�����2��ˮ�������ض����������õ�һ��
 ���γ�ˮ�������ӣ�
���γ�ˮ�������ӣ� �������ж��������̵�������
�������ж��������̵������� �������� ��
�������� ��| A����ԭ�ӵ��ӻ����ͷ����˸ı� | B��������״�����˸ı� |
| C�����Ļ�ѧ���ʷ����˸ı� | D�����еļ��Ƿ����˸ı� |
 �����ڵ�O-H�������Ӽ�ķ��»����������ǿ��������Ϊ_____________
�����ڵ�O-H�������Ӽ�ķ��»����������ǿ��������Ϊ_____________(��)�������������Ʋ�����Һ����Ҫԭ�ϣ�������Һ��һ�ֱ�����ɱ�������㷺Ӧ������ľ�������ͻ����ϡ�
(4)д��ͭԭ�Ӽ۵��Ӳ�ĵ����Ų�ʽ ����ͭͬһ���ڵĸ���Ԫ�صĻ�̬ԭ����������������ͭԭ����ͬ��Ԫ���� (��Ԫ�ط���)��
(5) ��ʢ������ͭˮ��Һ���Թ�����백ˮ�������γ�����������Ӱ�ˮ���������ܽ⣬�õ�����ɫ������Һ�����Ϲ����з��������ӷ�Ӧ����ʽΪ��
_______________________________��_______________________________��ʵ��ʱ�γɵ�����ɫ��Һ�е��������ڴ��ڵ�ȫ����ѧ�������� ��
(6)ʵ������м���
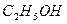 ��ɹ۲쵽��������ɫ
��ɹ۲쵽��������ɫ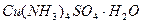 ���塣ʵ��������
���塣ʵ��������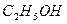 ��������__________________________________________________
��������__________________________________________________����13�֣������˱�ʾ�ģ�����ÿ��1�֣�
(1) �����������Ķ��У�
�����������Ķ��У�
��2��A��
��3��H-O������������»���
��4��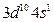 �� C��
�� C��
��5��Cu2+ + 2NH3��H2O = Cu(OH)2 + 2NH4+ ��2�֣�
Cu(OH)2 + 4 NH3 = [Cu(NH3)4]2+ + 2OH-��2�֣������ۼ�����λ������2�֣�
= [Cu(NH3)4]2+ + 2OH-��2�֣������ۼ�����λ������2�֣�
��6��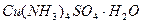 �����ӻ���������ڼ��Խ�С���Ҵ��У�����
�����ӻ���������ڼ��Խ�С���Ҵ��У�����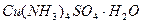 ���ܽ�ȣ������ھ���������2�֣�
���ܽ�ȣ������ھ���������2�֣�
(1)
 �����������Ķ��У�
�����������Ķ��У���2��A��
��3��H-O������������»���
��4��
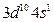 �� C��
�� C����5��Cu2+ + 2NH3��H2O = Cu(OH)2 + 2NH4+ ��2�֣�
Cu(OH)2 + 4 NH3
 = [Cu(NH3)4]2+ + 2OH-��2�֣������ۼ�����λ������2�֣�
= [Cu(NH3)4]2+ + 2OH-��2�֣������ۼ�����λ������2�֣���6��
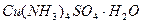 �����ӻ���������ڼ��Խ�С���Ҵ��У�����
�����ӻ���������ڼ��Խ�С���Ҵ��У�����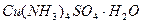 ���ܽ�ȣ������ھ���������2�֣�
���ܽ�ȣ������ھ���������2�֣���

��ϰ��ϵ�д�
�����Ŀ
 ��д��������NaOH��Һ��Ӧ�����ӷ���ʽ�� ��
��д��������NaOH��Һ��Ӧ�����ӷ���ʽ�� �� ����������F2����NH3��Ӧ�õ���NF3����________����.
����������F2����NH3��Ӧ�õ���NF3����________����.
 ���÷�Ӧ �����ǻ��ǣ�����-��ԭ��Ӧ
���÷�Ӧ �����ǻ��ǣ�����-��ԭ��Ӧ