��Ŀ����
����Ŀ���л������������صĽṹ����������
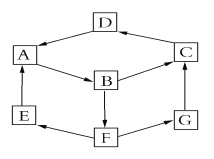
��1��������� �͵Ŀռ�ṹ������Ȳ����� �ͽṹ��
��2��������FeCl3��Һ���� ɫ����������Ũ���Ṳ�Ȼ�� ɫ�������������� ɫ����Щ���Գ����������ʵļ�������
��3�����ɵ����ʵİ����Ἰ������![]() -�����ᡣ����R����ʾ������
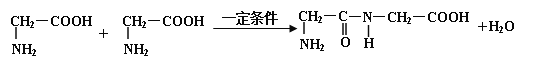
-�����ᡣ����R����ʾ������![]() -������Ľṹͨʽ�ɱ�ʾΪ �������������������һ�������������������һ�ֶ��ģ��÷�Ӧ�Ļ�ѧ����ʽΪ ��
-������Ľṹͨʽ�ɱ�ʾΪ �������������������һ�������������������һ�ֶ��ģ��÷�Ӧ�Ļ�ѧ����ʽΪ ��
���𰸡���1������������ֱ�� ��2������ ���� �� ��3��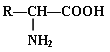
��������
�����������1������������������͵Ŀռ�ṹ������Ȳ�����ֱ���ͽṹ��
��2��������FeCl3��Һ������ɫ����������Ũ���Ṳ�Ȼ����ɫ����������������ɫ����Щ���Գ����������ʵļ�������
��3�����ɵ����ʵİ����Ἰ������![]() -�����ᡣ����R����ʾ������
-�����ᡣ����R����ʾ������![]() -������Ľṹͨʽ�ɱ�ʾΪ
-������Ľṹͨʽ�ɱ�ʾΪ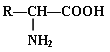 �������������������һ�����������������ȥ1����ˮ������һ�ֶ��ģ��÷�Ӧ�Ļ�ѧ����ʽΪ
�������������������һ�����������������ȥ1����ˮ������һ�ֶ��ģ��÷�Ӧ�Ļ�ѧ����ʽΪ ��
��

 ��������ܸ�ϰϵ�д�
��������ܸ�ϰϵ�д�����Ŀ������˶�Ա����ɵ��ܡ�������Ŀʱ���á�þ�ۡ����֣�������Ч����ij�֡�þ�ۡ��п��ܺ���Mg��MgO��Mg(OH)2��MgCO3�е�һ�ֻ����ֹ��壬ʵ��С�����ɷ�չ����̽����
��֪��MgO+2HCl=MgCl2+H2O MgCO3+2HCl=MgCl2+H2O+CO2��
��1��̽����þ�ۡ����Ƿ���Mg��MgCO3
��ȡ������Ʒ����ͼ��ʾ����ʵ�顣�۲쵽a�Թ��������ݲ�����b�Թ��в���������������ʯ��ˮ����ǣ���֤����þ�ۡ���һ������______��

��Ϊ֤����þ�ۡ����Ƿ���Mg��С����ȼ�ŵ�ľ������ͼ��b�Թܿ��Ϸ���ľ��Ϩ�𡣵�ͬѧ��ָ������ʵ�鲻�ܴ��ʵ��Ŀ�ģ���Ҫ��ͼ�еij���ʯ��ˮ�滻��ŨNaOH��Һ��Ŀ����______________��С�������ĺ��ʵ�鷽���ظ�����ʵ�飬�۲쵽b�Թ�������������֤����þ�ۡ���______________��
��2��̽����þ�ۡ����Ƿ���MgO��Mg(OH)2
��ʵ����̡�
��.��MgO��Mg(OH)2��MgCO3���ֹ���ֱ�������ʵ�顣�ֱ�ȡ0.5g���ֹ����ĩ��ÿ��ȡ��������ͼ2��ʾ��

��μ�����ͬ��������������ϡ����ֱ����ĩǡ����ʧ�����±��м�¼���ĵ�ͬŨ��ϡ���������������������ͬһ�����²ⶨ���ұ�����С�����1λ��
MgO | Mg(OH)2 | MgCO3 | |
����ϡ�������� /mL | 10.4 | 7.2 | 5.0 |
Mg(OH)2�����ᷢ���кͷ�Ӧ�Ļ�ѧ����ʽΪ_____________��
��.ȡ��þ�ۡ���Ʒ0.5 g����������ϡ��������ĩǡ���ܽ⡣��ʱ����ϡ��������ԼΪ5.3 mL��
��ʵ����������ۡ�
��þ�ۡ���ֻ����MgCO3��������____________________________________��
��ʵ�鷴˼��
Ϊȷ����þ�ۡ��ľ���ɷ֣�ͬѧ����Ϊ����Ҫ��������ʵ�飺�ֱ�ȡ0.5 g��þ�ۡ���0.5 g_________����������ϡ���ᣬ�ⶨ���ɵ���������ֱ�Ϊ119mL��140mL���ɴ˿�֪��þ�ۡ��к���MgCO3����������Ϊ___________��