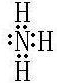��Ŀ����
�±���Ԫ�����ڱ��Ķ����ڲ��֣�������ĸ�ֱ��ʾһ��Ԫ�أ��ش��������⣺
|
a |
|
|
|||||
|
|
|
|
c |
e |
f |
g |
|
|
|
|
b |
d |
|
|
h |
|
��1��e��f��g����Ԫ����̬�⻯����ȶ���������ǿ��˳��Ϊ���û�ѧʽ��ʾ��__________��
��2��Ԫ��e����̬�⻯�����ʽΪ__________�����⻯����������������ˮ���ﷴӦ���ɵ�����������ѧ������Ϊ___________������_______����������ӻۣ���
��3��a��c����Ԫ�ؿ��γɶ��ֻ�������л�����A�IJ����Ǻ���һ������ʯ�ͻ�����չˮƽ�ı�־���� A�Ľṹ��ʽΪ____________����AΪԭ�ϣ����Ƶõ��������ϲ����������ϲ�Ʒ���÷�Ӧ����Ϊ_________________��A����H2O��Ӧ���ɻ�����B���÷�Ӧ����Ϊ__________________��B��CH3COOH��Ӧ�����й�����ζ�IJ���÷�Ӧ�Ļ�ѧ����ʽΪ_____________________________________________________ ���÷�Ӧ����Ϊ_____________��B����ͭΪ����ʱ��Ӧ�����д̼�����ζ�IJ���÷�Ӧ�Ļ�ѧ����ʽΪ_________________________________________________________��
��1��NH3��H2O��HF��2�� ���Ӽ������ۼ� ����
���Ӽ������ۼ� ����
��3��CH2��CH2 �Ӿ۷�Ӧ �ӳɷ�Ӧ
CH3COOH+CH3CH2OH CH3COOCH2CH3+H2O ȡ����Ӧ��������Ӧ����淴Ӧ��
CH3COOCH2CH3+H2O ȡ����Ӧ��������Ӧ����淴Ӧ��
2CH2CH2OH+O2 2CH3CHO+2H2O
2CH3CHO+2H2O
��������
�������������Ԫ�������ڱ��е����λ�ÿ�֪��a��hԪ�طֱ���H��Al��C��Si��N��O��F��Cl��
��1��ͬ�����������ҷǽ���������ǿ��ͬ�������϶��·ǽ������������ǽ�����Խǿ���⻯����ȶ���Խǿ����e��f��g����Ԫ����̬�⻯����ȶ���������ǿ��˳��ΪNH3��H2O��HF��
��2����Ԫ�ص��⻯���ǰ��������м��Լ��Ĺ��ۻ��������ʽ�� �����������ᷴӦ��������泥��û������������ӻ�����������Ӽ��ͼ��Լ���
�����������ᷴӦ��������泥��û������������ӻ�����������Ӽ��ͼ��Լ���
��3����ϩ�IJ����Ǻ���һ������ʯ�ͻ�����չˮƽ�ı�־����A����ϩ����ϩ����̼̼˫������A�Ľṹ��ʽΪCH2��CH2 ����ϩ�����Ӿ۷�Ӧ���ɾ���ϩ����ϩ�ܺ�ˮ�����ӳɷ�Ӧ�����Ҵ����Ҵ������ᷢ��������Ӧ����������������Ӧ�Ļ�ѧ����ʽ��CH3COOH+CH3CH2OH CH3COOCH2CH3+H2O���Ҵ��ڴ����������·���������Ӧ������ȩ����Ӧ�Ļ�ѧ����ʽ��2CH2CH2OH+O2
CH3COOCH2CH3+H2O���Ҵ��ڴ����������·���������Ӧ������ȩ����Ӧ�Ļ�ѧ����ʽ��2CH2CH2OH+O2 2CH3CHO+2H2O��
2CH3CHO+2H2O��
���㣺����Ԫ�����ڱ��Ľṹ�Լ�Ԫ�������ɵ�Ӧ�á�������ѧ�������д
�����������Ǹ߿��еij������ͣ������е��Ѷȵ����⡣���������ǿ�����ض�ѧ������֪ʶ�Ĺ��̺�ѵ�������������ѧ����������������Ӧ��������������Ҫ��Ԫ�ء�λ�������ԡ����߹�ϵ���ۺϿ��飬�Ƚ�ȫ�濼��ѧ���й�Ԫ���ƶ�֪ʶ���������֪ʶ��������������ѧ�������ʽṹ�����ʹ�ϵ�Լ�����Ԫ�������ɽ�����廯ѧ�����������

�±���Ԫ�����ڱ��Ķ����ڲ��֣�������ĸ�ֱ��ʾһ��Ԫ�أ��ش��������⣺
| a | | | |||||
| | | | c | e | f | g | |
| | | b | d | | | h | |
��1��e��f��g����Ԫ����̬�⻯����ȶ���������ǿ��˳��Ϊ���û�ѧʽ��ʾ��__________��
��2��Ԫ��e����̬�⻯�����ʽΪ__________�����⻯����������������ˮ���ﷴӦ���ɵ�����������ѧ������Ϊ___________������_______����������ӻۣ���
��3��a��c����Ԫ�ؿ��γɶ��ֻ�������л�����A�IJ����Ǻ���һ������ʯ�ͻ�����չˮƽ�ı�־���� A�Ľṹ��ʽΪ____________����AΪԭ�ϣ����Ƶõ��������ϲ����������ϲ�Ʒ���÷�Ӧ����Ϊ_________________��A����H2O��Ӧ���ɻ�����B���÷�Ӧ����Ϊ__________________��B��CH3COOH��Ӧ�����й�����ζ�IJ���÷�Ӧ�Ļ�ѧ����ʽΪ_____________________________________________________ ���÷�Ӧ����Ϊ_____________��B����ͭΪ����ʱ��Ӧ�����д̼�����ζ�IJ���÷�Ӧ�Ļ�ѧ����ʽΪ_________________________________________________________��
(14��)�±���Ԫ�����ڱ��Ķ����ڲ��֣�������ĸ�ֱ��ʾһ��Ԫ�ء���ش��������⣺
|
a |
|
|
|||||
|
|
|
|
b |
c |
d |
|
|
|
e |
|
f |
|
|
g |
|
|
(1) fԪ�������ڱ��е�λ���ǵ� ���ڵ� �塣
(2) e ��f��Ԫ������������ˮ�������Ӧ�Ļ�ѧ����ʽΪ
_________________________________________________
(3)e�ڿ�����ȼ�յIJ���������ѧ��������Ϊ__________��__________��
(4) ������ca3�ĵ���ʽΪ ��c��d����Ԫ���⻯����ȶ��Ը�ǿ���� _______�������ʵĻ�ѧʽ����