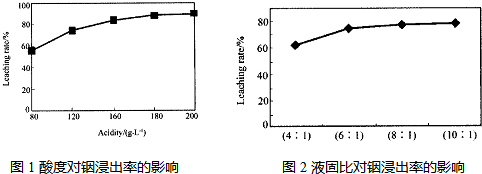
��Ŀ����
��2011?�ٴ���ģ�⣩���ԭ������ Ca��40 F��19
���Ϊ��ʽ���ڱ���һ���֣����еı�Ŵ�����Ӧ��Ԫ�أ�

��1��д������Ԫ�آ�ԭ�ӻ�̬ʱ�ĵ����Ų�ʽ
��2����Ԫ�آ�����γɵ���̬�����ˮ����������У�Ԫ�آڵ��ӻ���ʽΪ
��3���١���Ԫ�ص�p�Dz��������������ҵ�һ����������Ԫ��������
��4������ҵ����ȡ�ĵ����ǵ���������������Ȼ����ԭ��
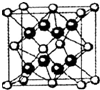
��5���ݺ͢��γɵ����ӻ������侧���ṹ��ͼ�������ӻ����ᄃ���ı߳�Ϊa cm��������ܶ���
���Ϊ��ʽ���ڱ���һ���֣����еı�Ŵ�����Ӧ��Ԫ�أ�

��1��д������Ԫ�آ�ԭ�ӻ�̬ʱ�ĵ����Ų�ʽ
[Ar]3d54s1
[Ar]3d54s1
����Ԫ�صķ�����Cr
Cr
����2����Ԫ�آ�����γɵ���̬�����ˮ����������У�Ԫ�آڵ��ӻ���ʽΪ
sp2
sp2
�ӻ��������ЦҼ���м�֮��Ϊ5��1
5��1
����3���١���Ԫ�ص�p�Dz��������������ҵ�һ����������Ԫ��������
��
��
�����������Ԫ���γɵĻ������У����ڷǼ��Է��ӵ���CO2
CO2
���ѧʽ����Ԫ�آ���Ԫ�آ��γɵ��⻯���У��е��ɸߵ���˳��ΪH2O��H2S
H2O��H2S
���ѧʽ������4������ҵ����ȡ�ĵ����ǵ���������������Ȼ����ԭ��
�����������Ӿ��壬����״̬���磬���Ȼ����Ƿ��Ӿ��壬����״̬������
�����������Ӿ��壬����״̬���磬���Ȼ����Ƿ��Ӿ��壬����״̬������
����5���ݺ͢��γɵ����ӻ������侧���ṹ��ͼ�������ӻ����ᄃ���ı߳�Ϊa cm��������ܶ���
4��78g?mol-1/NAa3cm3
4��78g?mol-1/NAa3cm3
g/cm3��ֻҪ���г���ʽ����
��������Ԫ�������ڱ��е�λ�ÿ�֪����ΪH����ΪC����ΪN����ΪO����ΪF����ΪAl����ΪS����ΪCa����ΪCr��
��1������ԭ�������������Ų�������������
��2�������γɵĻ�ѧ����������
��3������Ԫ�صķǽ����Լ������Ų���������һ�����ܣ����ݽṹ�������ӵļ��ԣ���������������⻯��ķе㣻
��4�����ݻ������������������ⷨ��ȡ������
��5���ݺ͢��γɵ����ӻ�����ΪCaF2���������ھ�����ռ�ݶ�������ģ����������������ɦ�=
�����㣮
��1������ԭ�������������Ų�������������
��2�������γɵĻ�ѧ����������
��3������Ԫ�صķǽ����Լ������Ų���������һ�����ܣ����ݽṹ�������ӵļ��ԣ���������������⻯��ķе㣻
��4�����ݻ������������������ⷨ��ȡ������
��5���ݺ͢��γɵ����ӻ�����ΪCaF2���������ھ�����ռ�ݶ�������ģ����������������ɦ�=
| m |
| V |
����⣺��Ԫ�������ڱ��е�λ�ÿ�֪����ΪH����ΪC����ΪN����ΪO����ΪF����ΪAl����ΪS����ΪCa����ΪCr��
��1��Ԫ�آ�ΪCr��ԭ������Ϊ24��������Ų�ʽΪ[Ar]3d54s1���ʴ�Ϊ��[Ar]3d54s1��Cr��
��2��������γɵ���̬�����ˮ���������ΪCH2=CH2��������ÿ��Cԭ���γɵĦҼ���Ϊ3����Cԭ�Ӳ�ȡsp2�ӻ��������к���4��C-H�Ҽ���1��C-C�Ҽ�������1���м��������ЦҼ���м�֮��Ϊ5��1���ʴ�Ϊ��sp2��5��1��
��3�����١���Ԫ���зǽ�����ǿ��ΪF�����ΪO��������p�Dz������������ӣ���p�Dz��������������ҵ�һ����������Ԫ������Ϊ�������������Ԫ���γɵĻ�������CO��CO2��������̼�Ľṹ�Գƣ�������ɵ������غϣ���Ϊ�Ǽ��Է��ӣ�����Ԫ�آ��γɵ��⻯����H2O�к���������е�ߣ��ʴ�Ϊ������CO2��H2O��H2S��
��4���������������Ӿ��壬����״̬���磬���Ȼ����Ƿ��Ӿ��壬����״̬�����磬�����õ���������ķ�����ұ����������
�ʴ�Ϊ�������������Ӿ��壬����״̬���磬���Ȼ����Ƿ��Ӿ��壬����״̬�����磻
��5���ݺ͢��γɵ����ӻ�����ΪCaF2���������ھ�����ռ�ݶ�������ģ����еĸ�����Ϊ8��
+6��
=4��
��������Ϊ
�����ӻ����ᄃ���ı߳�Ϊa cm�������Ϊa3��
�ɦ�=
��֪����=
=4��78 g?mol-1/NA a3cm3���ʴ�Ϊ��4��78 g?mol-1/NA a3cm3��
��1��Ԫ�آ�ΪCr��ԭ������Ϊ24��������Ų�ʽΪ[Ar]3d54s1���ʴ�Ϊ��[Ar]3d54s1��Cr��
��2��������γɵ���̬�����ˮ���������ΪCH2=CH2��������ÿ��Cԭ���γɵĦҼ���Ϊ3����Cԭ�Ӳ�ȡsp2�ӻ��������к���4��C-H�Ҽ���1��C-C�Ҽ�������1���м��������ЦҼ���м�֮��Ϊ5��1���ʴ�Ϊ��sp2��5��1��
��3�����١���Ԫ���зǽ�����ǿ��ΪF�����ΪO��������p�Dz������������ӣ���p�Dz��������������ҵ�һ����������Ԫ������Ϊ�������������Ԫ���γɵĻ�������CO��CO2��������̼�Ľṹ�Գƣ�������ɵ������غϣ���Ϊ�Ǽ��Է��ӣ�����Ԫ�آ��γɵ��⻯����H2O�к���������е�ߣ��ʴ�Ϊ������CO2��H2O��H2S��
��4���������������Ӿ��壬����״̬���磬���Ȼ����Ƿ��Ӿ��壬����״̬�����磬�����õ���������ķ�����ұ����������
�ʴ�Ϊ�������������Ӿ��壬����״̬���磬���Ȼ����Ƿ��Ӿ��壬����״̬�����磻
��5���ݺ͢��γɵ����ӻ�����ΪCaF2���������ھ�����ռ�ݶ�������ģ����еĸ�����Ϊ8��
| 1 |
| 8 |
| 1 |
| 2 |
��������Ϊ
| 78g/mol��4 |
| NA |
�ɦ�=
| m |
| V |
| ||
| a3 |
���������⿼��Ԫ�����ڱ���Ԫ�������ɣ���ϤԪ�������ڱ��е�λ���ǽ����Ĺؼ�����ע�����������еĻ�ѧ���������ṹͼ������ɣ��ѶȲ���

��ϰ��ϵ�д�
 ��Уͨ��֤��Ч��ҵϵ�д�
��Уͨ��֤��Ч��ҵϵ�д�
�����Ŀ

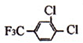 +
+
 +HCl
+HCl A�������۷�Ӧ
A�������۷�Ӧ +nH2O
+nH2O �ĺ�����������F3Cһ���ͱ�����ͬ���칹�干��
�ĺ�����������F3Cһ���ͱ�����ͬ���칹�干��