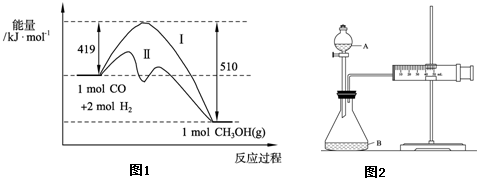
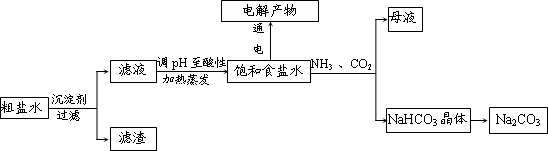
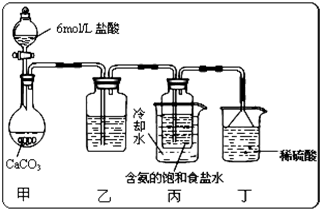
��Ŀ����
���ۺ�Ӧ�ã���ͼ��a��b��c��d��Ϊ���缫����ѡ���4��������Һ���±���

���������������������� A������������������������ B������������������������ C������������������������ D
���������������������� NaOH���������������� AgNO3���������������� H2Cl�������������������� NaCl
���������������������� CuSO4���������������� CuCl2���������������� AgNO3���������������� AgNO3
Ҫ����������ǣ�
�ٹ���һ��ʱ��ײ۵��ҺpH���������Ҳ۵��ҺpH�½���
��b��c�����ŵ����ӵ����ʵ�����ȡ���1��Ӧѡ�õĵ��Һ��________�顣
��2���ײ۵ĵ�ⷽ��ʽΪ________�Ҳ۵ĵ�ⷽ��ʽΪ________
KOH ������4OH����4e��=2H2O��O2��
������4H����4e ��=2H2��
��3���������ˮͬʱ������ⷴӦ�ĵ������Һ�У����ý������������Ρ������ý����ĺ������Σ�����ǰ����Һ��pH������С�����Ա���Ӧ�ǣ�CuSO4�ܷ�Ӧʽ��2CuSO![]() ��2H
��2H![]() O
O![]() 2Cu��2H
2Cu��2H![]() SO
SO![]() ��O
��O![]() ��
��
������
�㲦�����������Ҫ������ԭ�������ӷŵ�˳���֪ʶ�㣬����ѧ������˼ά���������ݵ����ɣ�������Һ��pH���ߵ���NaOH��NaCl��pH���͵���CuSO4��AgNO3��pH�����������CuCl2��pH���������ƣ������������ų�B��C������ס��ҳ��Ǵ����ģ���bΪ������cΪ������A��D����Һ�ĵ缫��ӦʽΪ�� A�飺 b����4OH�D�D4e��=O2����2H2O c����2Cu2����4e��=2Cu D��� b����2C1�D�D2e��=Cl2�� c����2Ag����2e��=2Ag ���������ų�A�����ԣ���ȷ����D�� ��2�����صĵ缫��Ӧʽ�ֱ�Ϊ���׳أ�2NaCl+2H
|

 ��ս�п�����ϵ�д�
��ս�п�����ϵ�д�