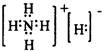��Ŀ����
ij������ˮ��Һ�����ܺ������������е������֣�K+��Al3+��Fe3+��Mg2+��Ba2+��NH4+��Cl-��CO32-��SO42-���ֱַ�ȡ100mL�����ȷ���Һ��������ʵ�飺
�ٵ�һ�ݼӹ���NaOH��Һ����ȣ��ռ���0.02mol���壬�������ɣ�ͬʱ�õ���Һ�ס�
�������Һ��ͨ�����CO2�����ɰ�ɫ���������������ˡ�ϴ�ӡ����պõ�1.02g���塣
�۵ڶ��ݼ�����BaCl2��Һ�����ɰ�ɫ��������������������ϴ�ӡ�����õ�11.65g���塣
����ʵ��ش��������⣺
��1���ɢٿ�֪���ڵ�����Ϊ ��Ũ���� mol��L-1��
�ɢڿ�֪���ڵ�����Ϊ ��Ũ���� mol��L-1��
�ɢۿ�֪���ڵ�����Ϊ ��Ũ���� mol��L-1��
��2������Һ��һ�������ڵ������� �������ӷ��ţ���
��3��ijͬѧͨ��������Ϊ����Һ��һ������K+������������ ��
(��10��)��1��NH4+ 0.2��Al3+ 0.2 �� SO42- 0.5 ��ÿ��1�֣���6�֣�
��2��Fe3+��Mg2+��Ba2+��CO32-��2�֣�
��3����֪��NH4+��Al3+�����������С��SO42-��������� �����ݵ���غ㣬һ����K+ ���ڣ�2�֣�
��������
�����������1����һ�ݼӹ���NaOH��Һ����ȣ��ռ���0.02mol���壬��˵�����ɵ�����һ���ǰ��������ʵ�����0.02mol�������һ������NH4+������ԭ���غ��֪��Ũ��NH4+��0.02mol��0.1L��0.2mol/L���������ɣ�ͬʱ�õ���Һ�ף���˵��һ��û��Fe3+��Mg2+�������Һ��ͨ�����CO2�����ɰ�ɫ���������������ˡ�ϴ�ӡ����պõ�1.02g���塣��˸ð�ɫ����ֻ��������������������Һ���к���ƫ�����ƣ�����ԭ��Һ�к���Al3+����Al3+����һ��û��CO32-������ԭ���غ��֪��Al3+Ũ����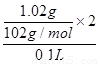 ��0.2mol/L���ڶ��ݼ�����BaCl2��Һ�����ɰ�ɫ��������������������ϴ�ӡ�����õ�11.65g���塣��˵���ð�ɫ����һ�������ᱵ������һ������SO42-�����һ��û��Ba2+������ԭ���غ��֪��SO42-��Ũ����
��0.2mol/L���ڶ��ݼ�����BaCl2��Һ�����ɰ�ɫ��������������������ϴ�ӡ�����õ�11.65g���塣��˵���ð�ɫ����һ�������ᱵ������һ������SO42-�����һ��û��Ba2+������ԭ���غ��֪��SO42-��Ũ����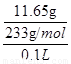 ��0.5mol/L��
��0.5mol/L��
��2������Һ��һ�������ڵ�������Fe3+��Mg2+��Ba2+��CO32-��
��3����֪��NH4+��Al3+�����������С��SO42-������������������ݵ���غ㣬һ����K+ ���ڡ�
���㣺�������ӹ��桢���ӵĶ��Լ���Ͷ�������