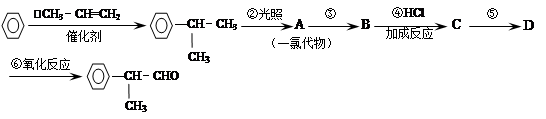��Ŀ����
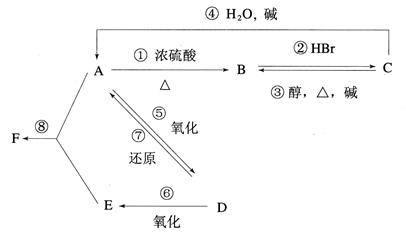
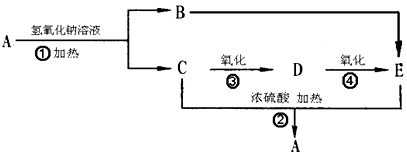
��16�֣�ij�л���A����C��H��O����Ԫ����ɣ���һ�������£���A����ת��Ϊ�л���B��C��D��E��C�ֿ���ת��ΪB��A�����ǵ�ת����ϵ���£�
��֪D�������ܶ���������22���������Է���������Ӧ��
��A~F�Ľṹ��ʽ����Ϊ____________��____________��____________��____________��____________��____________��
���ڢ�~���ת����������ȥ��Ӧ����____________�ӳɷ�Ӧ����____________ȡ����Ӧ����____________��
�ǣ���A~F�У�ѡ���ʵ�����ĸ��գ�����������ʳƷ��װ���ĵ�����__________����������������Cu(OH)2��Ӧ����_______________��Ŀǰ�ᳫ���ں����Ͱ�һ���������������������ȼ�ϵ���________________��
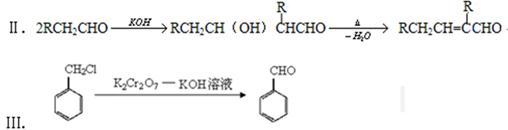
��4���ֱ�д���٢ݵĻ�ѧ����ʽ ��

��֪D�������ܶ���������22���������Է���������Ӧ��
��A~F�Ľṹ��ʽ����Ϊ____________��____________��____________��____________��____________��____________��
���ڢ�~���ת����������ȥ��Ӧ����____________�ӳɷ�Ӧ����____________ȡ����Ӧ����____________��
�ǣ���A~F�У�ѡ���ʵ�����ĸ��գ�����������ʳƷ��װ���ĵ�����__________����������������Cu(OH)2��Ӧ����_______________��Ŀǰ�ᳫ���ں����Ͱ�һ���������������������ȼ�ϵ���________________��
��4���ֱ�д���٢ݵĻ�ѧ����ʽ ��
��16�֣�������ʽÿ��2�֣�����ÿ��1�֣�
��1��CH3CH2OH CH2=CH2 CH3CH2Br CH3CHO CH3COOH CH3COOC2H5
��2���٢� �ڢ� �ܢ� ��3��B E A
��4�� ��
��
��1��CH3CH2OH CH2=CH2 CH3CH2Br CH3CHO CH3COOH CH3COOC2H5
��2���٢� �ڢ� �ܢ� ��3��B E A
��4��
 ��
��
���������D�������ܶ���������22��������Է���������44�����Է���������Ӧ������CH3CHO������D��ԭΪA��A����ΪD����AΪCH3CH2OH��D��һ������ΪE����EΪCH3COOH��FΪCH3COOC2H5��A������Ũ��������������B��B������HBr�ӳ�ΪC��C�ڴ��ͼ�����������ȥ��Ӧ����BΪCH2=CH2��CΪCH3CH2Br��
��1����A~F�Ľṹ��ʽ����ΪCH3CH2OH��CH2=CH2��CH3CH2Br��CH3CHO��CH3COOH��CH3COOC2H5��
��2�����ݷ�Ӧ���������жϣ�±��������ȥ��Ӧ�ڴ����������Ƽ��������£�������ȥ��Ӧ��Ũ������������£�Ϊ�٢ۣ�ȩ�Ļ�ԭ��Ӧ�����������ļӳɷ�Ӧ�����Լӳɷ�Ӧ���Тڢߣ�ȡ����Ӧ���Тܢࡣ
��3��ʳƷ��װ���IJ����Ǿ���ϩ����������ϩ����������������Cu(OH)2��Ӧ���Dz������Ϊȩ��ȩ�����Ƶ�������ͭ��Ӧ��Ҫ���ȣ����·�Ӧ��Ӧ�����CH3COOH���Ҵ�������Ŀǰ�ᳫ������������ȼ�ϣ����Ҵ������Ͱ�һ��������������ġ�
��4�������Ҵ�����ȥ��Ӧ��
 ��
�������Ҵ���������Ӧ��

�������л��ƶ�Ҫ���������Է�Ӧ��Ӱ�죬��±��������ȥ��Ӧ�ڴ����������Ƽ��������£�������ȥ��Ӧ��Ũ������������¡������ۺ���ǿ�����DZȽϻ��������ڽϼ��⡣

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ



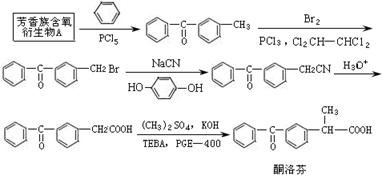
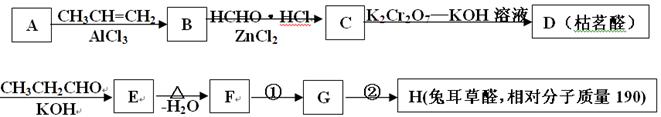
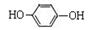 ����д��ʵ���ұ�����л��﷽���� ��
����д��ʵ���ұ�����л��﷽���� ��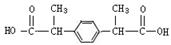 �ĺϳ�����ͼ�����Լ���ѡ����
�ĺϳ�����ͼ�����Լ���ѡ����
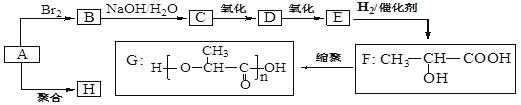
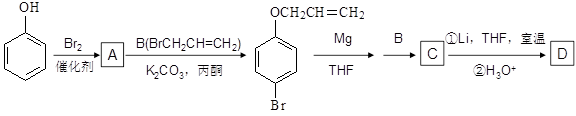
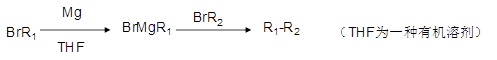
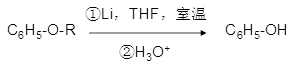
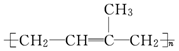
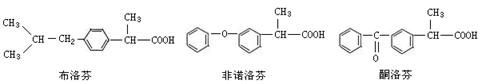
 �ǻ�����B��һ��ͬ���칹�壬��1H�˴Ź�������֤���û���������_____����ڲ�ͬ�Ļ�ѧ������
�ǻ�����B��һ��ͬ���칹�壬��1H�˴Ź�������֤���û���������_____����ڲ�ͬ�Ļ�ѧ������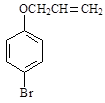 ���л���Ӧ����_________________��
���л���Ӧ����_________________��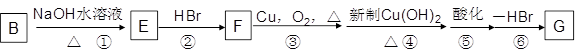
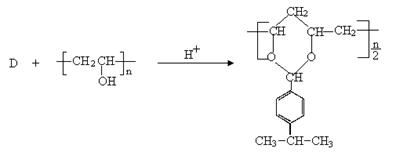
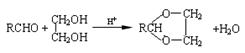
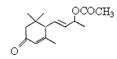 �Ƿ�Ϊͬ���칹�� ����ǡ�������������������
�Ƿ�Ϊͬ���칹�� ����ǡ�������������������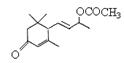 ��ͬ���칹���� �֣�д������һ�ֵĽṹ��ʽ ��
��ͬ���칹���� �֣�д������һ�ֵĽṹ��ʽ ��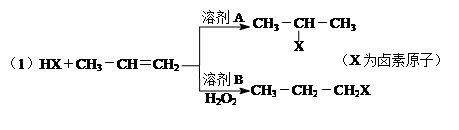
 �����ʣ���������һ�����ϡ�
�����ʣ���������һ�����ϡ�