��Ŀ����
(����Ԥ����)��֪25 ��ʱ������ʵĵ���ƽ�ⳣ����
Ka(CH3COOH)��1.8��10��5��Ka(HSCN)��0.13��
(1)��20 mL 0.10 mol��L��1 CH3COOH��Һ��20 mL 0.10 mol��L��1��HSCN��Һ�ֱ���0.10 mol��L��1��NaHCO3��Һ��Ӧ��ʵ���ò���CO2�������(V)��
ʱ��t�Ĺ�ϵ��ͼ��ʾ��
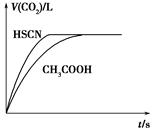
��Ӧ��ʼʱ��������Һ����CO2���������Բ�ͬ��ԭ����________����Ӧ������������Һ��c(SCN��)______c(CH3COO��)(���������������)��
(2)2.0��10��3 mol��L��1�������ˮ��Һ�У�������ҺpH(���Ե���ʱ����仯)�����ƽ����ϵ��c(F��)��c(HF)����ҺpH�Ĺ�ϵ��ͼ��ʾ����25 ��ʱ��HF����ƽ�ⳣ��ΪKa(HF)��________(��ʽ��ֵ)��
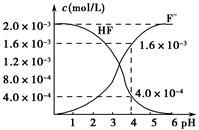
(3)��������CaF2�ܶȻ�����ΪKsp��1.5��10��10����4.0��10��3 mol��L��1 HF��Һ��4.0��10��4 mol��L��1��CaCl2��Һ�������ϣ�������ҺpH��4(���Ե���ʱ��Һ����仯)���Է�����Ϻ��Ƿ��г������ɣ�________(��С���û�С�)���������ɣ�_____________________________________��
Ka(CH3COOH)��1.8��10��5��Ka(HSCN)��0.13��
(1)��20 mL 0.10 mol��L��1 CH3COOH��Һ��20 mL 0.10 mol��L��1��HSCN��Һ�ֱ���0.10 mol��L��1��NaHCO3��Һ��Ӧ��ʵ���ò���CO2�������(V)��
ʱ��t�Ĺ�ϵ��ͼ��ʾ��

��Ӧ��ʼʱ��������Һ����CO2���������Բ�ͬ��ԭ����________����Ӧ������������Һ��c(SCN��)______c(CH3COO��)(���������������)��
(2)2.0��10��3 mol��L��1�������ˮ��Һ�У�������ҺpH(���Ե���ʱ����仯)�����ƽ����ϵ��c(F��)��c(HF)����ҺpH�Ĺ�ϵ��ͼ��ʾ����25 ��ʱ��HF����ƽ�ⳣ��ΪKa(HF)��________(��ʽ��ֵ)��

(3)��������CaF2�ܶȻ�����ΪKsp��1.5��10��10����4.0��10��3 mol��L��1 HF��Һ��4.0��10��4 mol��L��1��CaCl2��Һ�������ϣ�������ҺpH��4(���Ե���ʱ��Һ����仯)���Է�����Ϻ��Ƿ��г������ɣ�________(��С���û�С�)���������ɣ�_____________________________________��
(1)Ka(HSCN)��Ka(CH3COOH)����Һ��c(H��)��HSCN��CH3COOH��
c(H��)��Ӧ���ʿ졡��
(2)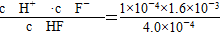 ��4��10��4
��4��10��4
(3)�С���Һ�����ӻ�Qc��c(Ca2��)��c2(F��)��5.12��10��10��Ksp(CaF2)��1.5��
10��10
c(H��)��Ӧ���ʿ졡��
(2)
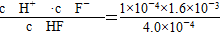 ��4��10��4
��4��10��4(3)�С���Һ�����ӻ�Qc��c(Ca2��)��c2(F��)��5.12��10��10��Ksp(CaF2)��1.5��
10��10
(1)��Ka(CH3COOH)��Ka(HSCN)������֪����ͬ���ʵ���Ũ����ҺHSCN�����Ա�CH3COOHǿ��HSCN��Һ��c(H��)�����Է�Ӧ��ʼʱHSCN��Һ��NaHCO3��Һ��Ӧ�����ʿ졣��Ӧ��������������Һ�ֱ�ΪCH3COONa��Һ��NaSCN��Һ������HSCN�����Ա�CH3COOHǿ����SCN����ˮ��̶ȱ�CH3COO����������c(SCN��)��c(CH3COO��)��(2)��pH��4ʱ����ͼ���֪��c(F��)��1.6��10��3 mol��L��1��c(HF)��4.0��10��4mol��L��1��c(H��)��1��10��4mol��L��1������Ka(HF)�� ���ɡ�(3)��pH��4ʱ����Һ�е�c(F��)��1.6��10��3 mol��L��1����Һ��c(Ca2��)��2.0��10��4 mol��L��1����Һ�����ӻ�Qc��c(Ca2��)��c2(F��)��5.12��10��10��Ksp(CaF2)��1.5��10��10�����г���������
���ɡ�(3)��pH��4ʱ����Һ�е�c(F��)��1.6��10��3 mol��L��1����Һ��c(Ca2��)��2.0��10��4 mol��L��1����Һ�����ӻ�Qc��c(Ca2��)��c2(F��)��5.12��10��10��Ksp(CaF2)��1.5��10��10�����г���������
 ���ɡ�(3)��pH��4ʱ����Һ�е�c(F��)��1.6��10��3 mol��L��1����Һ��c(Ca2��)��2.0��10��4 mol��L��1����Һ�����ӻ�Qc��c(Ca2��)��c2(F��)��5.12��10��10��Ksp(CaF2)��1.5��10��10�����г���������
���ɡ�(3)��pH��4ʱ����Һ�е�c(F��)��1.6��10��3 mol��L��1����Һ��c(Ca2��)��2.0��10��4 mol��L��1����Һ�����ӻ�Qc��c(Ca2��)��c2(F��)��5.12��10��10��Ksp(CaF2)��1.5��10��10�����г���������
��ϰ��ϵ�д�
�����Ŀ
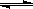 2H++Cl��+ClO��
2H++Cl��+ClO��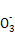 ��ClO-
��ClO-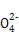 ��SCN-
��SCN-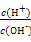 =1012����Һ��:N
=1012����Һ��:N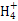 ��Al3+��S
��Al3+��S