��Ŀ����
����Ŀ��(1)����ͼ��ʾ��������ַ�Ӧ��
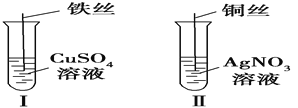
�٢��з�����Ӧ�����ӷ���ʽΪ___________________________________��
�ڢ���ͭ˿�Ϲ۲쵽��������______________________________________
�۽�Ϣ�����ʵ�������֪Fe2����Cu2����Ag������������ǿ������˳��Ϊ__________��
(2)������Cl2ͨ��FeCl2����Һ�У���Ӧ�����ӷ���ʽΪ2Fe2����Cl2===2Fe3����2Cl���������ʵ˵�����л�ԭ�Ե����ӻ�ԭ��ǿ��Ϊ___________________
(3) NaHCO3��Һ����������ʯ��ˮ��Ӧ�����ӷ���ʽ_____________________________
(4)ijһ��Ӧ��ϵ���з�Ӧ��������ﹲ5�����ʣ�S��H2S��HNO3��NO��H2O��
��д����Ӧ����ʽ���������ת�Ʒ�����Ŀ______________________________________
������Ӧ����ʽ��ת����0.3 mol���ӣ������������������______________________
���𰸡�Fe+Cu2��==Fe2��+Cu �������ɫ Ag����Cu2����Fe2�� Fe2����Cl�� HCO3-+OH-+Ca2+== H2O+CaCO3�� 3H2S+2HNO3 ==3S+2NO��+4H2O 4.8g
��������
��1������������ͭ�����û���Ӧ��
��ͭ�Ļ����Ա���ǿ������ͭ�ܰ����ӿ���������Һ���û�������ʹ��ɫ��ͭ˿�������ɫ��
�۸��ݻ�ԭ�ԣ���ԭ�����ڻ�ԭ����.�����ԣ�������������������������
��2�����ݻ�ԭ���Ļ�ԭ�Դ��ڻ�ԭ����Ļ�ԭ�ԣ�
��3��NaHCO3��Һ����������ʯ��ˮ��Ӧ������CaCO3��NaOH��
��4������������ԭ��Ӧ�У����ϼ�������������ȵĹ��������
��1��������ͭ���ã���������ͭ�����û���Ӧ�����ӷ���ʽΪ��Fe+Cu2��==Fe2��+Cu��
�������Fe+Cu2��==Fe2��+Cu��
��ͭ�Ļ����Ա���ǿ������ͭ�ܰ����ӿ���������Һ���û�����������ͭ˿�Ϲ۲쵽�������ǣ���ɫ��ͭ��������ɫ�������ţ�ʹ��ɫ��ͭ˿�������ɫ��
�����Ϊ���������ɫ��
�ۢ�����ӷ���ʽΪFe![]() Cu2+=Fe2+
Cu2+=Fe2+![]() Cu��������ӷ���ʽΪCu
Cu��������ӷ���ʽΪCu![]() 2Ag+=2Ag
2Ag+=2Ag![]() Cu2+������������ǿ����ϵ�ɵã�Fe2����Cu2����Ag������������ǿ������˳��Ϊ��Ag����Cu2����Fe2����
Cu2+������������ǿ����ϵ�ɵã�Fe2����Cu2����Ag������������ǿ������˳��Ϊ��Ag����Cu2����Fe2����
�������Ag����Cu2����Fe2����
��2����Ӧ2Fe2����Cl2===2Fe3����2Cl�����о��л�ԭ�Ե�������Fe2����Cl�������ݻ�ԭ���Ļ�ԭ�Դ��ڻ�ԭ����Ļ�ԭ�ԣ����л�ԭ�ԣ�Fe2��![]() Cl����
Cl����
�������Fe2��![]() Cl����
Cl����
��3��NaHCO3��Һ����������ʯ��ˮ��Ӧ������CaCO3��NaOH�����ӷ���ʽΪ��HCO3-+OH-+Ca2+== H2O+CaCO3����
�������HCO3-+OH-+Ca2+== H2O+CaCO3����
��4����S��H2S��HNO3��NO��H2O�У��������ʷ�����������Ӧ��HNO3����ǿ�����ԣ�����HNO3����������H2S���л�ԭ���ǻ�ԭ���� S���������NO�ǻ�ԭ����ʷ�Ӧ�Ļ�ѧ����ʽΪ��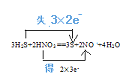 ��
��
�����ݵ�ʧ��������ȵĹ�����ת����0.3 mol���ӣ�������������ʵ���ΪXmol�����У�6:3=0.3��X�����X=0.15mol,��������S������Ϊ��0.15mol![]() 32g/mol=4.8g��
32g/mol=4.8g��
�������4.8.

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�


