��Ŀ����
����Ŀ��ʵ�� �����ܼ��š� �͡���̼���á���һ����Ҫ���������ν�CO2ת��Ϊ�����õ���Դ��Ŀǰ��ҵ����һ�ַ�������CO2������ȼ�ϼ״���һ�������·�����Ӧ��CO2(g)��3H2(g)![]() CH3OH(g)��H2O(g)����ͼ1��ʾ�÷�Ӧ����������(��λΪkJ��mol��1)�ı仯��
CH3OH(g)��H2O(g)����ͼ1��ʾ�÷�Ӧ����������(��λΪkJ��mol��1)�ı仯��
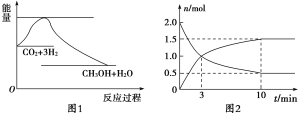
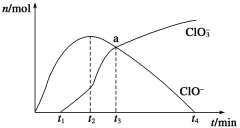
(1)Ϊ̽����Ӧԭ�����ֽ�������ʵ�飬�����Ϊ2 L���ܱ������У�����2 mol CO2��8 mol H2��һ�������·�����Ӧ��CO2(g)��3H2(g)![]() CH3OH(g)��H2O(g)�����CO2��CH3OH(g)��Ũ����ʱ��仯����ͼ2��ʾ��
CH3OH(g)��H2O(g)�����CO2��CH3OH(g)��Ũ����ʱ��仯����ͼ2��ʾ��
�ٴӷ�Ӧ��ʼ��ƽ�⣬CH3OH��ƽ����Ӧ����v(CH3OH) ��________��
�ڸ÷�Ӧ��ƽ�ⳣ������ʽK��________��
(2)830 �棬��Ӧ��ƽ�ⳣ��K��1���ں��ݷ�Ӧ���з���������Ӧ�����±��е����ʵ���Ͷ�뷴Ӧ��������������Ӧ������е���________(�A����B����C����D��)��
���� | A | B | C | D |
n(CO2) | 3 | 1 | 3 | 1 |
n(H2) | 2 | 2 | 4 | 2 |
n(CH3OH) | 1 | 2 | 3 | 0.5 |
n(H2O) | 4 | 2 | 3 | 2 |
(3)25 �棬1.01��105Paʱ��16gҺ̬�״���ȫȼ�գ����ָ���ԭ״̬ʱ���ų�363.3 kJ��������д���÷�Ӧ���Ȼ�ѧ����ʽ______________________________��
���𰸡�0.075 mol��L��1��min��1![]() ACDCH3OH(l)��
ACDCH3OH(l)��![]() O2(g)===CO2(g)��2H2O(l)��H����726.6 kJ/mol
O2(g)===CO2(g)��2H2O(l)��H����726.6 kJ/mol
��������
(1)��ͼ2��v(CH3OH)��![]() ��0.075 mol��L��1��min��1���÷�Ӧƽ�ⳣ���ı���ʽK=
��0.075 mol��L��1��min��1���÷�Ӧƽ�ⳣ���ı���ʽK=![]() ���ʴ�Ϊ����0.075 mol��L��1��min��1��
���ʴ�Ϊ����0.075 mol��L��1��min��1��![]() ��(2)�����㣬 B��Qc��1�������ƶ���A��C��D��Qc��1�������ƶ����ʴ�ΪACD����3��16gҺ̬�״���ȫȼ�գ����ָ���ԭ״̬ʱ���ų�363.3 kJ����1mol�״�ȼ�շų�������Ϊ32g��16g��363.3KJ=726.7KJ���ʷ�Ӧ���Ȼ�ѧ��Ӧ����ʽΪ: CH3OH(l)��
��(2)�����㣬 B��Qc��1�������ƶ���A��C��D��Qc��1�������ƶ����ʴ�ΪACD����3��16gҺ̬�״���ȫȼ�գ����ָ���ԭ״̬ʱ���ų�363.3 kJ����1mol�״�ȼ�շų�������Ϊ32g��16g��363.3KJ=726.7KJ���ʷ�Ӧ���Ȼ�ѧ��Ӧ����ʽΪ: CH3OH(l)��![]() O2(g)===CO2(g)��2H2O(l)��H����726.6 kJ/mol��
O2(g)===CO2(g)��2H2O(l)��H����726.6 kJ/mol��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ�������£���һԪ��HA��Һ��KOH��Һ�������ϣ�ʵ���������±���
ʵ���� | ��ʼŨ��/(mol��L��1) | ��Ӧ���� Һ��pH | |
[HA] | [KOH] | ||
�� | 0.1 | 0.1 | 9 |
�� | x | 0.2 | 7 |
�����жϲ���ȷ����(����)
A. ʵ��ٷ�Ӧ�����Һ�У�[K��]>[A��]>[OH��]>[H��]
B. ʵ��ٷ�Ӧ�����Һ�У�[OH��]��[K��]��[A��]��![]() mol��L��1
mol��L��1
C. ʵ��ڷ�Ӧ�����Һ�У�[A��]��[HA]>0.1 mol��L��1
D. ʵ��ڷ�Ӧ�����Һ�У�[K��]��[A��]>[OH��]��[H��]