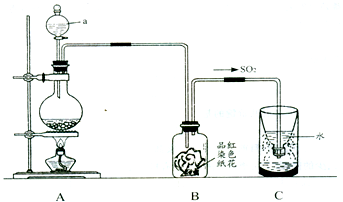
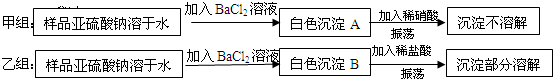
��Ŀ����
�����Ǵ�����Ⱦ��֮һ����������SO2���ĺ����ǿ�����������һ����Ҫָ�꣮ij��ȤС��ͬѧȡ�ս������ȵ糧��������ˮ����ʵ�飺
�ٲ�ø���ˮ��Ʒ��pHΪ4.73��
������ˮ��Ʒ�еμ�BaCl2��Һ���а�ɫ�������ɣ�
��ÿ��1h��ͨ��pH�Ʋⶨ��ˮ��Ʒ��pH����õĽ�����±���
����������Ϣ���ش��������⣺
��1��������ˮ��pHΪ5.6��ƫ���ԣ�������Ϊ
��2�������������ݱ仯������Ϊ�γ���һ�仯��ԭ����
��3����ȤС��ͬѧȡijһʱ�ε�������ˮV L������0.5mol?L-1��Ba��OH��2��Һ�����ٲ�������ʱ��ǡ������60.00mL Ba��OH��2��Һ��
����㣺�ٸ�V L��ˮ���ܽ�SO2���������״����
�����ɳ������������Χ��д��������̣���
�ٲ�ø���ˮ��Ʒ��pHΪ4.73��
������ˮ��Ʒ�еμ�BaCl2��Һ���а�ɫ�������ɣ�
��ÿ��1h��ͨ��pH�Ʋⶨ��ˮ��Ʒ��pH����õĽ�����±���
| �ⶨʱ��/h | 0 | 1 | 2 | 3 | 4 |
| ��ˮ��Ʒ��pH | 4.73 | 4.62 | 4.56 | 4.55 | 4.55 |
��1��������ˮ��pHΪ5.6��ƫ���ԣ�������Ϊ
�����е�CO2������ˮ
�����е�CO2������ˮ
����2�������������ݱ仯������Ϊ�γ���һ�仯��ԭ����
H2SO3������е�O2����ת��ΪH2SO4��ʹ��ˮ��������ǿ��PH��С
H2SO3������е�O2����ת��ΪH2SO4��ʹ��ˮ��������ǿ��PH��С
����3����ȤС��ͬѧȡijһʱ�ε�������ˮV L������0.5mol?L-1��Ba��OH��2��Һ�����ٲ�������ʱ��ǡ������60.00mL Ba��OH��2��Һ��
����㣺�ٸ�V L��ˮ���ܽ�SO2���������״����
�����ɳ������������Χ��д��������̣���
��������1�������д���CO2����ˮʹ��ˮ�����ԣ�������ˮ��pHֵԼ����5.6��
��2�������ŷų�����SO2����������ˮ������H2SO3��H2SO3����ˮ���½����������е��������������������
H2SO4��2H2SO3+O2=2H2SO4���Ӷ�ʹ��ˮ��������ǿ��
��3����������ijɷ֣�H2SO3��H2SO4������H2SO3��Ba��OH��2��H2SO4��Ba��OH��2���ɵ�n��SO2��=n��H2SO3��+n��H2SO4��=n[Ba��OH��2]�����SO2����������ݼ����������ɫ����ȫ��ΪBaSO3�Լ���ɫ����ȫ��ΪBaSO4ʱ�����������������ɫ�����ķ�Χ��
��2�������ŷų�����SO2����������ˮ������H2SO3��H2SO3����ˮ���½����������е��������������������
H2SO4��2H2SO3+O2=2H2SO4���Ӷ�ʹ��ˮ��������ǿ��
��3����������ijɷ֣�H2SO3��H2SO4������H2SO3��Ba��OH��2��H2SO4��Ba��OH��2���ɵ�n��SO2��=n��H2SO3��+n��H2SO4��=n[Ba��OH��2]�����SO2����������ݼ����������ɫ����ȫ��ΪBaSO3�Լ���ɫ����ȫ��ΪBaSO4ʱ�����������������ɫ�����ķ�Χ��
����⣺��1�������д���CO2����ˮ����̼��ʹ��ˮ�����ԣ�������ˮ��pHֵԼ����5.6��
�ʴ�Ϊ�������е�CO2������ˮ��
��2����ˮ��pHֵ��С��ԭ���������ŷų�����SO2����������ˮ������H2SO3��H2SO3����ˮ���½����������е��������������������H2SO4��.2H2SO3+O2=2H2SO4���Ӷ�ʹ��ˮ��������ǿ��
�ʴ�Ϊ��H2SO3������е�O2����ת��ΪH2SO4��ʹ��ˮ��������ǿ��PH��С��
��3��������ijɷ�ΪH2SO3��H2SO4������H2SO3��Ba��OH��2��H2SO4��Ba��OH��2���ɵ�n��SO2��=n��H2SO3��+n��H2SO4��=n[Ba��OH��2]=0.5mol?L-1��60.00mL��10-3L?mL-1=0.0300mol������V��SO2��=0.0300mol��22.4L?mol-1=0.672L
�𣺸�V L��ˮ���ܽ�SO2�����Ϊ0.672L��
�������ɳ����������Ϊw
����ɫ����ȫ��ΪBaSO3����������Ϊ0.0300mol��217g?mol-1=6.51g
����ɫ����ȫ��ΪBaSO4����������Ϊ0.0300mol��233g?mol-1=6.99g
���ԣ�6.51g��w��6.99g
�����ɳ������������ΧΪ6.51g��w��6.99g��
�ʴ�Ϊ�������е�CO2������ˮ��
��2����ˮ��pHֵ��С��ԭ���������ŷų�����SO2����������ˮ������H2SO3��H2SO3����ˮ���½����������е��������������������H2SO4��.2H2SO3+O2=2H2SO4���Ӷ�ʹ��ˮ��������ǿ��
�ʴ�Ϊ��H2SO3������е�O2����ת��ΪH2SO4��ʹ��ˮ��������ǿ��PH��С��
��3��������ijɷ�ΪH2SO3��H2SO4������H2SO3��Ba��OH��2��H2SO4��Ba��OH��2���ɵ�n��SO2��=n��H2SO3��+n��H2SO4��=n[Ba��OH��2]=0.5mol?L-1��60.00mL��10-3L?mL-1=0.0300mol������V��SO2��=0.0300mol��22.4L?mol-1=0.672L
�𣺸�V L��ˮ���ܽ�SO2�����Ϊ0.672L��
�������ɳ����������Ϊw
����ɫ����ȫ��ΪBaSO3����������Ϊ0.0300mol��217g?mol-1=6.51g
����ɫ����ȫ��ΪBaSO4����������Ϊ0.0300mol��233g?mol-1=6.99g
���ԣ�6.51g��w��6.99g
�����ɳ������������ΧΪ6.51g��w��6.99g��
������������Ҫ�����˸��ݻ�ѧ����ʽ���м��㣬ͬʱ���ݼ����ж������������

��ϰ��ϵ�д�
�����Ŀ
�����Ǵ�����Ⱦ��֮һ����������SO2���ĺ����ǿ�����������һ����Ҫָ�꣮ij��ȤС��ͬѧȡ�ս������ȵ糧��������ˮ����ʵ�飺
�ٲ�ø���ˮ��Ʒ��pHΪ4.73��
������ˮ��Ʒ�еμ�BaCl2��Һ���а�ɫ�������ɣ�
��ÿ��1h��ͨ��pH�Ʋⶨ��ˮ��Ʒ��pH����õĽ�����±���
| �ⶨʱ��/h | 0 | 1 | 2 | 3 | 4 |
| ��ˮ��Ʒ��pH | 4.73 | 4.62 | 4.56 | 4.55 | 4.55 |
��1��������ˮ��pHΪ5.6��ƫ���ԣ�������Ϊ______��
��2�������������ݱ仯������Ϊ�γ���һ�仯��ԭ����______��
��3����ȤС��ͬѧȡijһʱ�ε�������ˮV L������0.5mol?L-1��Ba��OH��2��Һ�����ٲ�������ʱ��ǡ������60.00mL Ba��OH��2��Һ��
����㣺�ٸ�V L��ˮ���ܽ�SO2���������״����
�����ɳ������������Χ��д��������̣���
�����Ǵ�����Ⱦ��֮һ����������SO2���ĺ����ǿ�����������һ����Ҫָ�꣮ij��ȤС��ͬѧȡ�ս������ȵ糧��������ˮ����ʵ�飺
�ٲ�ø���ˮ��Ʒ��pHΪ4.73��
������ˮ��Ʒ�еμ�BaCl2��Һ���а�ɫ�������ɣ�
��ÿ��1h��ͨ��pH�Ʋⶨ��ˮ��Ʒ��pH����õĽ�����±���
����������Ϣ���ش��������⣺
��1��������ˮ��pHΪ5.6��ƫ���ԣ�������Ϊ______��
��2�������������ݱ仯������Ϊ�γ���һ�仯��ԭ����______��
��3����ȤС��ͬѧȡijһʱ�ε�������ˮV L������0.5mol?L-1��Ba��OH��2��Һ�����ٲ�������ʱ��ǡ������60.00mL Ba��OH��2��Һ��
����㣺�ٸ�V L��ˮ���ܽ�SO2���������״����
�����ɳ������������Χ��д��������̣���
�ٲ�ø���ˮ��Ʒ��pHΪ4.73��
������ˮ��Ʒ�еμ�BaCl2��Һ���а�ɫ�������ɣ�
��ÿ��1h��ͨ��pH�Ʋⶨ��ˮ��Ʒ��pH����õĽ�����±���
| �ⶨʱ��/h | 1 | 2 | 3 | 4 | |
| ��ˮ��Ʒ��pH | 4.73 | 4.62 | 4.56 | 4.55 | 4.55 |
��1��������ˮ��pHΪ5.6��ƫ���ԣ�������Ϊ______��
��2�������������ݱ仯������Ϊ�γ���һ�仯��ԭ����______��
��3����ȤС��ͬѧȡijһʱ�ε�������ˮV L������0.5mol?L-1��Ba��OH��2��Һ�����ٲ�������ʱ��ǡ������60.00mL Ba��OH��2��Һ��
����㣺�ٸ�V L��ˮ���ܽ�SO2���������״����
�����ɳ������������Χ��д��������̣���