��Ŀ����
����Ŀ����ҩ������ҽѧ��Խ��Խ�ܵ���ע����ҩҩ����˪����Ҫ�ɷ�As2O3�����������Ƽ���Ѫ����Ϊ�ˣ��鼰�仯�������ȡ�ٴ������ע��
��1��As��33��Ԫ�أ����������ڱ��е�λ����________________��
��2��NԪ�طǽ����Ա�Asǿ������˵����ȷ����_________________________��
��NH3�����ȶ��Ա�AsH3�� ��HNO3�����Ա�H3AsO4ǿ ��N��ԭ�Ӱ뾶��As��ԭ�Ӱ뾶С
��3��������ͼд��As2O5�ֽ�ΪAs2O3���Ȼ�ѧ����ʽ__________________��

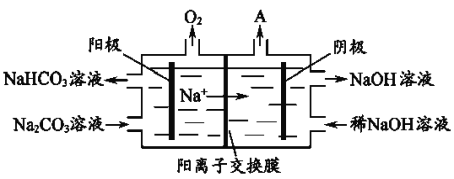
��4��������֪�����Դ����飨As2S3����������ȡAs2O3����ȡ�Ĺ������̼�ͼ���£�

��As2S3��Na3AsS3�е�S��Ϊ-2�ۣ���������з����ķ�Ӧ______����ǡ����ǡ���������ԭ��Ӧ��
�����̢��ϵ�в�������________________________________________________��
��д�����̢�����ӷ���ʽ__________________________________________________�����̢��У�����Խǿ��As2O3�IJ���Խ�ߣ��������ԭ��______________________________��
���𰸡��������ڣ� VA�� �ڢ� As2O5(s) === As2O3(s) + O2(g) ��H= + 295.4 kJ��mol-1 ���� ������ϴ������� 2AsO4 3��+ 2SO2 + 2H+ === As2O3 + 2SO42�� + H2O ����Խǿ�����ʵ������Ի���ԭ�Կ�����ǿ������������Ũ��ƽ�������ƶ��ٽ���Ӧ�Ľ��У����������As2O3�IJ���
��������
����As��Ԫ�����ڱ��е�λ���ж�As�Ļ�ѧ���ʣ�����Ԫ�������ɷ�����𣻸����Ȼ�ѧ��Ӧ����ʽ����д����������
(1)As��ԭ�Ӻ�������Ų�ͼ�ǣ� ![]() ����4�����Ӳ㣬�������5�����ӣ�λ�ڵ�������VA�壬�ʴ�Ϊ����������VA�壻
����4�����Ӳ㣬�������5�����ӣ�λ�ڵ�������VA�壬�ʴ�Ϊ����������VA�壻
(2)NԪ�طǽ����Ա�Asǿ��˵��N���⻯�����ȶ��Ա�As���⻯���ȶ������ȶ��ԣ�NH3>AsH3���ʢٴ���NԪ�ص�����������ˮ��������Ա�AsԪ�ص�����������ˮ���������ǿ�������ԣ�HNO3>H3AsO4���ʢ���ȷ��N��As��ͬ����Ԫ�أ�ͬ����Ԫ�أ����ϵ��£��ǽ�����������ԭ�Ӱ뾶�����ʢ���ȷ���ʴ�Ϊ�� �ڢۣ�
(3) ����ͼ����ʾ����֪As2O5�ֽ�ΪAs2O3���Ȼ�ѧ����ʽAs2O5��s���TAs2O3��s��+O2��g�� ��H=-619.2 kJmol-1+(-914.6 kJmol-1)=+295.4kJmol-1���ʴ�Ϊ��As2O5(s) === As2O3(s) + O2(g) ��H= + 295.4 kJ��mol-1��
(4) �ټ��������û��Ԫ�ػ��ϼ۵ı仯�����Ըù��̲���������ԭ��Ӧ���ʴ�Ϊ�����ǣ�
�ڹ��̢�Ϊ��Һ�����壬��Ҫ����Ũ���ᾧ�����ˣ�ϴ�ӣ�����ʴ�Ϊ��Ũ���ᾧ�����ˣ�ϴ�ӣ���ɣ�
�۹��̢�����Ӧ�����ӷ���ʽΪ2AsO43-+2SO2+2H+�TAs2O3+2SO42-+H2O������Խǿ��������Ũ��Խ������������Ũ��ƽ�������ƶ��ٽ���Ӧ�Ľ��У����������As2O3�IJ��ʣ��ʴ�Ϊ������Խǿ�����ʵ������Ի���ԭ�Կ�����ǿ������������Ũ��ƽ�������ƶ��ٽ���Ӧ�Ľ��У����������As2O3�IJ��ʡ�

 �¿α�����Ķ�ѵ��ϵ�д�
�¿α�����Ķ�ѵ��ϵ�д�