��Ŀ����
����Ŀ��ij����С������ʵ��������100mL1.0mol��L��1��������Һ��Ȼ�����о�����ͭ������ķ�Ӧ�����
��1������100mL1.0mol��L��1��������Һ����Ҫ���������ձ�������������Ͳ����ͷ�ι��⣬����Ҫ�IJ���������______________________��
��2������10 mol��L��1��Ũ����������������Һ����Ҫ��ȡŨ����������_______mL��
��3�����ƹ����У���������������ȷ�����в���������Ũ��ƫС����__________��
A������ҡ�Ⱥ���Һ����ڿ̶��ߣ�����ˮ���̶���
B������ʱ��������ƿ�Ŀ̶���
C������Һת������ƿ��û��ϴ���ձ��Ͳ��������ͽ��ж��ݲ���
D������ƿ������ˮϴ�Ӻ�δ���
��4������С��ͬѧ���Ƶõ�������ͭƬ������װ���з�Ӧ����Ӧ���ڹ۲쵽���Թ��е�Һ��a��Ϊ��ɫ���Թ��Ϸ���dz����ɫ������֡�
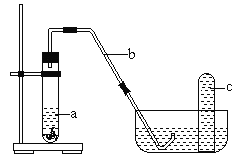
�� ��Ӧ����Һ��a��Ϊ��ɫ�����ڷ�Ӧ������____________���ѧʽ����
�� ���º���ɫ�������Թ�c����ɫ��ʧ�ķ�Ӧ����ʽΪ____________________��
�� ���ܷ�ͨ����ʵ���������ó�1.0mol��L��1��������ͭƬ��Ӧֱ�����ɺ���ɫNO2�Ľ��ۣ�_________������������������������������ __________________________��
���𰸡� 100mL����ƿ 10 AC Cu(NO3)2 3NO2+H2O=2HNO3+NO ���� װ�����п�����2NO+O2=2NO2����ʹ��ʼû��NO2��ֻ��NO����Ҳ���ܱ�ɺ���ɫ��
����������1������100mL1.0mol��L��1��������Һ����Ҫ��������������ձ�������������Ͳ����ͷ�ι��⣬����Ҫ100mL����ƿ��
��2������Ҫ10 mol��L��1��Ũ����VmL������ϡ�Ͷ��ɿ�֪��10 mol��L��1��VmL=100mL��1.0mol��L��1����V=10��
��3��A������ҡ�Ⱥ���������ˮ��������Һ����ڿ̶��ߣ��ټ�ˮ���̶��ߣ�������Һ���ƫ��Ũ��ƫ�ͣ���A��ȷ��B������ʱ��������ƿ�Ŀ̶��ߣ�Һ���ڿ̶����£���Һ���ƫ�ͣ�Ũ��ƫ��B����C������Һת������ƿ��û��ϴ���ձ��Ͳ��������ͽ��ж��ݲ���������ƿ������ƫ�ͣ�Ũ��ƫ�ͣ���C��ȷ��D������ʱ��Ҫ������ƿ������ˮ������ƿ������ˮϴ�Ӻ�δ��ɣ���ʵ������Ӱ�죬��D����ΪAC��
��4���� Cu�ܽ�����������Cu(NO3)2 ��ʹ��Һ��Ϊ��ɫ��
�� ����ɫ����NO2�ܽ���ˮ���������NO����ɫ��ʧ��������Ӧ�Ļ�ѧ����ʽΪ3NO2+H2O=2HNO3+NO��
�� Һ���Ϸ������е����������������ɵ�NOΪNO2����ʹ��ʼû��NO2��ֻ��NO����Ҳ���ܱ�ɺ���ɫ���ʲ��ܽ�����������ֱ���жϷ�Ӧ���ɺ���ɫNO2��
