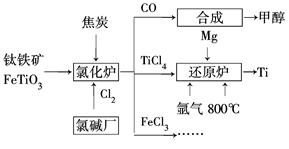
��Ŀ����
2013�����ȫ�����ض�����ж�������ʮ������������ɡ������족����Ҫ��Դ֮һ������β����ȼúβ���ŷų����Ĺ���С������
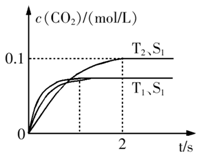
����β����������Ҫԭ��Ϊ��2NO(g)+2CO(g)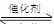 2CO2+N2�����ܱ������з����÷�Ӧʱ��c(CO2)���¶�(T)�������ı����(S)��ʱ��(t)�ı仯��������ͼ��ʾ���ݴ��жϣ�
2CO2+N2�����ܱ������з����÷�Ӧʱ��c(CO2)���¶�(T)�������ı����(S)��ʱ��(t)�ı仯��������ͼ��ʾ���ݴ��жϣ�
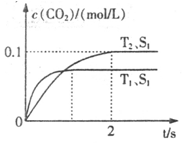
��1���÷�ӦΪ ��Ӧ(����ȡ������ȡ�������T2�¶��£�0~2s�ڵ�ƽ����Ӧ���ʣ�v(N2)= ����2�����������������һ��ʱ�����������������ѧ��Ӧ���ʡ��������ı����S1>S2���ڴ���ϻ��� c(CO2)��T1��S2�����´ﵽƽ������еı仯���ߡ�
��3��ij���л�������t1���£�����㶨���ܱ������У������崫��������˲�ͬʱ���NO��CO��Ũ�ȣ��������ݼ��±���CO2��N2����ʼŨ��Ϊ0����
t1��ʱ�÷�Ӧ��ƽ�ⳣ��K= ��ƽ��ʱNO���������Ϊ ��
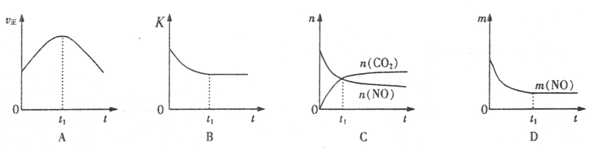
��4�����÷�Ӧ�ھ��ȡ����ݵ��ܱ���ϵ�н��У�����ʾ��ͼ��ȷ����˵����Ӧ�ڽ��е�t1ʱ�̴ﵽƽ��״̬���� (����ţ�������ͼ��v����K��n��m�ֱ��ʾ����Ӧ���ʡ�ƽ�ⳣ�������ʵ�����������
��5��úȼ�ղ���������Ҳ�������������CH4����ԭNOX�������������������Ⱦ��
��֪��CH4(g)+2NO2(g) = N2 (g)+CO2 (g)+2H2O(g) ��H=-867��0kJ ? mol-1
2NO2 (g) N2O4 (g) ��H=-56��9kJ ? mol-1
N2O4 (g) ��H=-56��9kJ ? mol-1
H2O(g) = H2O(l) ��H=-44��0kJ ? mol-1
д��CH4����ԭN2O4 (g)����N2 (g)��CO2 (g)��H2O(l)���Ȼ�ѧ����ʽ ��
����β����������Ҫԭ��Ϊ��2NO(g)+2CO(g)
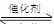 2CO2+N2�����ܱ������з����÷�Ӧʱ��c(CO2)���¶�(T)�������ı����(S)��ʱ��(t)�ı仯��������ͼ��ʾ���ݴ��жϣ�
2CO2+N2�����ܱ������з����÷�Ӧʱ��c(CO2)���¶�(T)�������ı����(S)��ʱ��(t)�ı仯��������ͼ��ʾ���ݴ��жϣ� 
��1���÷�ӦΪ ��Ӧ(����ȡ������ȡ�������T2�¶��£�0~2s�ڵ�ƽ����Ӧ���ʣ�v(N2)= ����2�����������������һ��ʱ�����������������ѧ��Ӧ���ʡ��������ı����S1>S2���ڴ���ϻ��� c(CO2)��T1��S2�����´ﵽƽ������еı仯���ߡ�
��3��ij���л�������t1���£�����㶨���ܱ������У������崫��������˲�ͬʱ���NO��CO��Ũ�ȣ��������ݼ��±���CO2��N2����ʼŨ��Ϊ0����
| ʱ��/s | 0 | 1 | 2 | 3 | 4 | 5 |
| c(NO)/xl0-4mol L-1 | 10��0 | 4��50 | 2��50 | 1��50 | 1��00 | 1��00 |
| c(CO)/xl0-3mol L-1 | 3��60 | 3��05 | 2��85 | 2��75 | 2��70 | 2��70 |
t1��ʱ�÷�Ӧ��ƽ�ⳣ��K= ��ƽ��ʱNO���������Ϊ ��
��4�����÷�Ӧ�ھ��ȡ����ݵ��ܱ���ϵ�н��У�����ʾ��ͼ��ȷ����˵����Ӧ�ڽ��е�t1ʱ�̴ﵽƽ��״̬���� (����ţ�������ͼ��v����K��n��m�ֱ��ʾ����Ӧ���ʡ�ƽ�ⳣ�������ʵ�����������

��5��úȼ�ղ���������Ҳ�������������CH4����ԭNOX�������������������Ⱦ��
��֪��CH4(g)+2NO2(g) = N2 (g)+CO2 (g)+2H2O(g) ��H=-867��0kJ ? mol-1
2NO2 (g)
 N2O4 (g) ��H=-56��9kJ ? mol-1
N2O4 (g) ��H=-56��9kJ ? mol-1H2O(g) = H2O(l) ��H=-44��0kJ ? mol-1
д��CH4����ԭN2O4 (g)����N2 (g)��CO2 (g)��H2O(l)���Ȼ�ѧ����ʽ ��
��1������ 0��025 mol/(L��s) ����2�֣���4�֣�
��2����ͼ����T1S1�·�����㲻�䡢�յ������ߺɣ��������ɣ���2�֣�
��3��5000 L/mol ��2�֣� 2��41% (2��)
��4��B D ����2�֣���ѡ��1�֣���ѡ���÷֣�
��5��CH4(g)+N2O4(g)�TN2(g)+CO2(g)+2H2O(g) D H�T ��898��1kJ/mol ��2�֣�
��������������ݡ��ȹ���ƽ����ֵ��ԭ��֪��T1��T2������T1�����¿�֪��������̼�ĺ��������ͣ���֪�������¶ȷ�Ӧ�����ƶ�����������Ƿ��ȵġ���ѧ��Ӧ���ʵ��ڵ�λʱ���ڷ�Ӧ�����������Ũ�ȵı仯������˿���ȷ���ö�����̼��ʾ�Ļ�ѧ��Ӧ���ʾ�Ϊ��0.050 mol/(L��s)�������ݻ�ѧ��Ӧ����֮�ȵ��ڻ�ѧ������֮�ȣ���֪���õ�����ʾ�Ļ�ѧ��Ӧ���ʾ�Ϊ��0.025 mol/(L��s)��������������ֻ������Ӧ���ʣ�����Ӱ�������̼�ĺ�������ˣ�ֻ�Ƿ�Ӧ�ﵽƽ���ʱ���ӳ�������Ӱ�������̼������
�� 2NO(g) + 2CO(g)
 2CO2 + N2
2CO2 + N2��ʼŨ�ȣ�10��3mol/L 3.6��10��3mol/L 0 0
ת��Ũ�ȣ�9.0��10��4mol/L 9.0��10��4mol/L 9.0��10��4mol/L 4.5��10��4mol/L
ƽ��Ũ�ȣ�10��4mol/L 2.7��10��3mol/L 9.0��10��4mol/L 4.5��10��4mol/L
���ƽ�ⳣ����
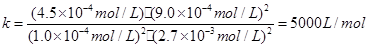
��駣�ƽ��ʱ����ȵ������ʵ���֮�ȣ�����Ũ��֮�ȣ�����ƽ��ʱNO���������Ϊ��
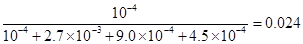

��Aͼ������Ӧ���ʷ�Ӧ��ʼ���Ǽ�С�ģ�����Bͼ�б�ʾ���ǻ�ѧƽ�ⳣ����ʱ��ı仯����Ϊ�Ǿ��ȵģ�����Ӧ���Ƿ��ȵģ��������ŷ�Ӧ�Ľ��У��ų�������ɢ����ȥ��ʹ��Ӧ�����ƶ���ʹ��ƽ�ⳣ����С������ijһ��ʱ�̴ﵽƽ��״̬����ȷ��Cͼ����t1����Ե����ʵ������ڱ仯������ƽ��״̬������Dͼ��һ��������������t1ʱ���������䣬һ����һ��ƽ��״̬����ȷ��
�� ��CH4(g)+2NO2(g) = N2 (g)+CO2 (g)+2H2O(g) ��H=-867��0kJ ? mol-1
��2NO2 (g)
 N2O4 (g) ��H=-56��9kJ ? mol-1
N2O4 (g) ��H=-56��9kJ ? mol-1��H2O(g) = H2O(l) ��H=-44��0kJ ? mol-1
�٣��ڣ��ۡ�2��CH4(g)+N2O4(g)�TN2(g)+CO2(g)+2H2O(g) D H�T ��898��1kJ/mol

��ϰ��ϵ�д�
�����Ŀ
 CO2(g)+4H2(g)
CO2(g)+4H2(g)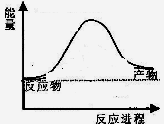
 2NH3(g)��H����92.2kJ��mol��1
2NH3(g)��H����92.2kJ��mol��1
 2NH3(g) ��H=��92��4kJ/mol
2NH3(g) ��H=��92��4kJ/mol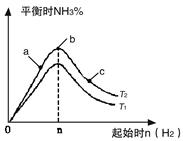
 4NO2(g)��O2(g) ��H��0�±�Ϊ��Ӧ��T1�¶��µIJ���ʵ������
4NO2(g)��O2(g) ��H��0�±�Ϊ��Ӧ��T1�¶��µIJ���ʵ������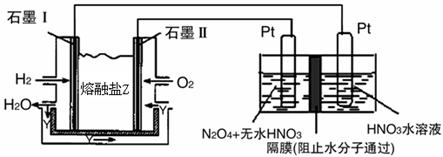
 CO2(g)+2H2O(l)����H1=-Q1
CO2(g)+2H2O(l)����H1=-Q1 CO(g)��H2(g)��C(s)��CO(g)��H2(g)��ȫȼ�յ��Ȼ�ѧ����ʽΪ��
CO(g)��H2(g)��C(s)��CO(g)��H2(g)��ȫȼ�յ��Ȼ�ѧ����ʽΪ�� O2(g)=H2O(g)����H����242.0 kJ��mol��1
O2(g)=H2O(g)����H����242.0 kJ��mol��1