��Ŀ����
����Ŀ����֪����1molH2(g)�е�H��H����Ҫ����436kJ����������1molI2(g)�е�I��I����Ҫ����151kJ����������HI(g)�е�1molH��I���ܷų�299kJ����������˵����ȷ����
A.1molH2(g)��1molI2(g)��������Ϊ587kJ
B.H2(g)��I2(s)![]() 2HI(g)��H����11kJ��mol��1
2HI(g)��H����11kJ��mol��1
C.HI(g)![]()
![]() H2(g)��
H2(g)��![]() I2(g)��H��+5.5kJ��mol��1
I2(g)��H��+5.5kJ��mol��1
D.H��H����I��I����������
���𰸡�C
��������
A. ���������ɻ����1mol��ѧ���ͷŻ����յ��������������ʾ��е���������A����
B. H2(g)��I2(g)![]() 2HI(g) ��H��436kJ+151kJ��299kJ��2= -11kJ��mol��1��I2(g)����������I2(s)������H2(g)��I2(s)
2HI(g) ��H��436kJ+151kJ��299kJ��2= -11kJ��mol��1��I2(g)����������I2(s)������H2(g)��I2(s)![]() 2HI(g)��H>��11kJ��mol��1����B����
2HI(g)��H>��11kJ��mol��1����B����
C. H2(g)��I2(g)![]() 2HI(g) ��H��436kJ+151kJ��299kJ��2=- 11kJ��mol��1������ HI(g)
2HI(g) ��H��436kJ+151kJ��299kJ��2=- 11kJ��mol��1������ HI(g)![]()
![]() H2(g)��
H2(g)��![]() I2(g)��H��+5.5kJ��mol��1����C��ȷ��
I2(g)��H��+5.5kJ��mol��1����C��ȷ��
D. H��H���ļ��ܴ���I��I��������I��I���������ѣ���D����
ѡC��

����Ŀ��ij��ȤС���ô�CuO(��������FeO)�Ʊ��Ȼ�ͭ����(CuCl2��2H2O)����һ���ⶨ���ȣ������������£�

�����Ϣ���£�
���Ȼ�ͭ��ˮ��Һ�нᾧʱ����15�����µõ���ˮ���15��25.7��õ���ˮ���26��42��õ���ˮ���42�����ϵõ�һˮ���100��õ���ˮ�
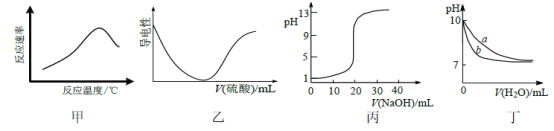
�ڽ��������γ��������������pH��Χ��
�������� | pH | |
��ʼ���� | ��ȫ���� | |
Fe3+ | 1.5 | 2.8 |
Fe2+ | 5.5 | 8.3 |
Cu2+ | 5.2 | 6.4 |
(1)����II�����Թ�����˫��ˮ��Ŀ����___��
(2)����III����Ҫ�õ����������IJ�����
a.�������ܽ� b.��pH=3.0 c.��pH=7.0 d.ϴ�� e.���ˡ�
�����������������ȷ˳��________(�������ظ�ʹ��)��
(3)����IV��װ��ͼ��ͼ��
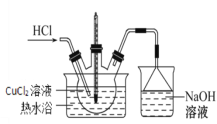
�ٳ���ͨ��HCl��������________��
�ڼ�������Ũ��������Һ������ٵ�ԭ����![]() ʱ������ˮԡ�¶ȿ�����_______ʱ�������壬���ˣ�ϴ�ӣ����
ʱ������ˮԡ�¶ȿ�����_______ʱ�������壬���ˣ�ϴ�ӣ����
�۹�������������˵������������________��
A.����ʱ�����ò�����ά�����ֽ
B.ϴ�Ӿ���ʱӦ�ȹر�ˮ��ͷ���ټ���ϴ�Ӽ�
C.�������ʱ���Ͽ�ˮ�ú�����ƿ֮����ܣ��ٹر�ˮ��ͷ
D.Ϊ�õ�������CuCl2��2H2O�����˲��õ��¸���
(4)������ӵ��������ⶨCuCl2��2H2O��Ʒ�Ĵ��ȣ��������£�ȡ0.4000g��������ˮ���������KI���壬��ַ�Ӧ�����ɰ�ɫCuI���������뼸�ε�����Һ��ָʾ������0.1000mol��L-1Na2S2O3 ����Һ�ζ�������ζ��յ�ʱ������Na2S2O3����Һ20.00mL��(�ζ���ӦΪI2+2S2O32-=S4O62-+2I-)
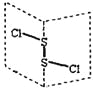
��д�����ɰ�ɫCuI���������ӷ���ʽ________��
�ڸ���Ʒ��CuCl2��2H2O����������Ϊ________��