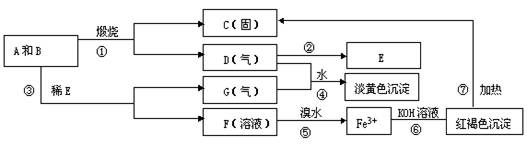
��Ŀ����
�о���ѧϰС�����SO2���Ʊ�������̽��ʵ�顣
 ��1�����ݷ�ӦNa2SO3(��)��H2SO4(Ũ)��Na2SO4��SO2��H2O���Ʊ�SO2���塣
��1�����ݷ�ӦNa2SO3(��)��H2SO4(Ũ)��Na2SO4��SO2��H2O���Ʊ�SO2���塣
 �������м�ͼ���ڴ���ķ����л����Ʊ����ռ�SO2��ʵ��װ��(���Լ�)ʾ��ͼ��
�������м�ͼ���ڴ���ķ����л����Ʊ����ռ�SO2��ʵ��װ��(���Լ�)ʾ��ͼ��

 ��ʵ������У�ʹ�÷�Һ©���μ�Ũ����IJ����� ��
��ʵ������У�ʹ�÷�Һ©���μ�Ũ����IJ����� ��
 ��2����SO2����ֱ�ͨ��������Һ�У�
��2����SO2����ֱ�ͨ��������Һ�У�
 ��Ʒ����Һ�������� ��
��Ʒ����Һ�������� ��
 ����ˮ��Һ�������� ��
����ˮ��Һ�������� ��
 ��������Һ�������� ��
��������Һ�������� ��
 ��3����һС����ʵ���з��֣�SO2����������������º���ʵ������ܲ����ԣ����ֲ��������������⡣�����Ʋ���ܵ�ԭ��˵����Ӧ����֤���������Բ���������
��3����һС����ʵ���з��֣�SO2����������������º���ʵ������ܲ����ԣ����ֲ��������������⡣�����Ʋ���ܵ�ԭ��˵����Ӧ����֤���������Բ���������
 ��ԭ�� ����֤���� ��
��ԭ�� ����֤���� ��
 ��ԭ�� ����֤���� ��
��ԭ�� ����֤���� ��
 ��ԭ�� ����֤���� ��
��ԭ�� ����֤���� ��
 ��1�����ݷ�ӦNa2SO3(��)��H2SO4(Ũ)��Na2SO4��SO2��H2O���Ʊ�SO2���塣
��1�����ݷ�ӦNa2SO3(��)��H2SO4(Ũ)��Na2SO4��SO2��H2O���Ʊ�SO2���塣 �������м�ͼ���ڴ���ķ����л����Ʊ����ռ�SO2��ʵ��װ��(���Լ�)ʾ��ͼ��
�������м�ͼ���ڴ���ķ����л����Ʊ����ռ�SO2��ʵ��װ��(���Լ�)ʾ��ͼ��

 ��ʵ������У�ʹ�÷�Һ©���μ�Ũ����IJ����� ��
��ʵ������У�ʹ�÷�Һ©���μ�Ũ����IJ����� �� ��2����SO2����ֱ�ͨ��������Һ�У�
��2����SO2����ֱ�ͨ��������Һ�У� ��Ʒ����Һ�������� ��
��Ʒ����Һ�������� �� ����ˮ��Һ�������� ��
����ˮ��Һ�������� �� ��������Һ�������� ��
��������Һ�������� �� ��3����һС����ʵ���з��֣�SO2����������������º���ʵ������ܲ����ԣ����ֲ��������������⡣�����Ʋ���ܵ�ԭ��˵����Ӧ����֤���������Բ���������
��3����һС����ʵ���з��֣�SO2����������������º���ʵ������ܲ����ԣ����ֲ��������������⡣�����Ʋ���ܵ�ԭ��˵����Ӧ����֤���������Բ��������� ��ԭ�� ����֤���� ��
��ԭ�� ����֤���� �� ��ԭ�� ����֤���� ��
��ԭ�� ����֤���� �� ��ԭ�� ����֤���� ��
��ԭ�� ����֤���� ����1����
 �ڴ�Һ©���ϿڵĻ�����������Һ©���������������μ�
�ڴ�Һ©���ϿڵĻ�����������Һ©���������������μ�
 ��2������Һ��ɫ �� ��Һ��ɫ �� ��dz��ɫ��Һ������Һ����ǣ�
��2������Һ��ɫ �� ��Һ��ɫ �� ��dz��ɫ��Һ������Һ����ǣ�
 ��3����Na2SO3����ȡ�����������Թ��У�����������ˮ�����Һ���ȵ�������ϡ���ᣬ�ٵ���BaCl2��Һ�а�ɫ�������ɣ���֤����Na2SO3�������
��3����Na2SO3����ȡ�����������Թ��У�����������ˮ�����Һ���ȵ�������ϡ���ᣬ�ٵ���BaCl2��Һ�а�ɫ�������ɣ���֤����Na2SO3�������

 �ڴ�Һ©���ϿڵĻ�����������Һ©���������������μ�
�ڴ�Һ©���ϿڵĻ�����������Һ©���������������μ� ��2������Һ��ɫ �� ��Һ��ɫ �� ��dz��ɫ��Һ������Һ����ǣ�
��2������Һ��ɫ �� ��Һ��ɫ �� ��dz��ɫ��Һ������Һ����ǣ� ��3����Na2SO3����ȡ�����������Թ��У�����������ˮ�����Һ���ȵ�������ϡ���ᣬ�ٵ���BaCl2��Һ�а�ɫ�������ɣ���֤����Na2SO3�������
��3����Na2SO3����ȡ�����������Թ��У�����������ˮ�����Һ���ȵ�������ϡ���ᣬ�ٵ���BaCl2��Һ�а�ɫ�������ɣ���֤����Na2SO3������� ��1�����ݷ�Ӧԭ������ĿҪ���Ʊ�SO2���ռ������ǵ�SO2�ж��ȿ��Ի���װ��ͼ����2��SO2����ͨ��Ʒ����ҺƷ����ɫ��ͨ����ˮ����������ˮ����ԭ��Br-����ɫ��ͨ��Na2S��Һ����2S2-+SO2 +4H+
��1�����ݷ�Ӧԭ������ĿҪ���Ʊ�SO2���ռ������ǵ�SO2�ж��ȿ��Ի���װ��ͼ����2��SO2����ͨ��Ʒ����ҺƷ����ɫ��ͨ����ˮ����������ˮ����ԭ��Br-����ɫ��ͨ��Na2S��Һ����2S2-+SO2 +4H+ 3S��+2H2O����dz��ɫ���ǡ���3���������������⣬����ԭ�ֱ�Ϊ��Na2SO3���ʲ�����Na2SO4������HCl��BaCl2��֤�����õIJ���ŨH2SO4��������ŨH2SO4����ˮ�Ե�������֤��
3S��+2H2O����dz��ɫ���ǡ���3���������������⣬����ԭ�ֱ�Ϊ��Na2SO3���ʲ�����Na2SO4������HCl��BaCl2��֤�����õIJ���ŨH2SO4��������ŨH2SO4����ˮ�Ե�������֤��
��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ