��Ŀ����
ʵ��������ҩƷ
���������ơ�����̨����20cm��ľ������Ե�������۱ʡ���ͷ�ιܡ���˿��ͭ˿����ˮ�Ҵ���0.2mol?L-1��ϡ���ᡣ
����ʵ�鷽������������
1.����ͼ����װ�ã�����������δ��������
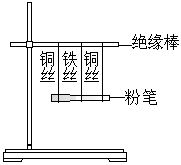
2.�ڷ۱��ϵμ���ˮ�Ҵ����õ����Ƶ�����ͷ�ֱ�Ӵ���ͭ˿��Ȼ����������ͷ�Ӵ�ͭ˿����˿��
3.�ڷ۱��ϵμ�0.2mol?L-1��ϡ���ᣬ�ظ�����2��
���������������۲쵽��ʵ������ش��������⣺
��1���ڲ���2�У������Ƶ�ָ���Ƿ�ƫת���������������Է���ԭ��
��2���ڲ���3�У��������Ƶ�����ͷ�Ӵ���ͭ˿ʱ��ָ���Ƿ�ƫת�����������������Ӵ�ͭ˿����˿ʱ��ָ���Ƿ�ƫת���������������Է���ԭ��
��3����������2�е���ˮ�Ҵ���Ϊ������Һ���۲쵽��������ͬ���Է���ԭ��
��4����ʵ��˵�����ԭ��ر���߱��������У�
��_________________________��
��_________________________��
��_________________________��
��5������װ�ÿ����γ�ԭ��ص���_____��

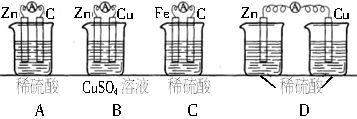
��6���������ԭ��ص����������Է�ӦFe+Cu2+=Fe2++Cu���һ��ԭ��ء�
��ѡ�õĵ������Һ��ϡ���ᡢ����ͭ��Һ���Ȼ�ͭ��Һ��
��ѡ�õĵ缫���ϣ�пƬ��ͭƬ����Ƭ��ʯī��
�������ϣ�______________���缫��Ӧʽ��________________________________��
�������ϣ�______________���缫��Ӧʽ��________________________________��
�������Һ��___________��
��1����ƫת��������������Ϊ�Ҵ�Ϊ�ǵ������Һ�����ܹ���ԭ���
��2�����Ӵ���ͭ˿ʱ��ָ�벻ƫת�����������������缫����ͬ���ϣ��������ԭ��أ����Ӵ�ͭ˿����˿ʱ��ָ��ƫת���е��������������ԭ���
��3������ͬ������Ϊ�������Һ����ͭ˿����˿�������ԭ��أ�������ָ��ƫת
��4���������缫������һ����Խϻ��ã���һ����Բ����ã��Ե���������ֱ�ӽӴ��� ���е������Һ ���γɱպϻ�·
��5��ABC
��6����Ƭ��Fe-2e-=Fe2+��ͭƬ��ʯī��Cu2++2e-=Cu������ͭ��Һ���Ȼ�ͭ��Һ
�����������

��1�������������IJ���������
������ҩƷ�У�a���Ȼ����Һb����ʯ��c��Ũ��ˮd��Ũ����e���Ȼ���
������������������ҩƷ��ѡ�ú�����ţ�����±���
| �Ʊ�NH3 | ���� | ��ѧ����ʽ | ||
| ���� | ҩƷ | ���� | ҩƷ | |
��3���罫��������ͨ����з�̪��AgNO3��Һ�У�����Ϊ
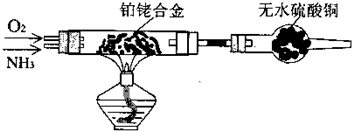
��������ͭ��ͭ����ɵĻ���ijͬѧ������ͼ��ʾװ�ã�ͨ���ⶨ�����������ʵ��ǰ��U�������仯��ȷ�������������ͭ������������
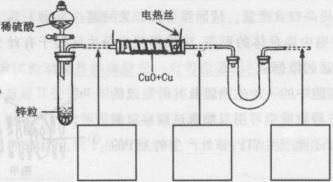 |
�ش��������⣺
(1)U�ι��п��Լ���������� (�����)��
A��Ũ![]() B����ˮ����ͭ��ĩ C����ˮ�Ȼ��ƿ���
B����ˮ����ͭ��ĩ C����ˮ�Ȼ��ƿ���
(2)���в��谴ʵ�����˳��ӦΪ (����ĸ)��
a��ֹͣͨ������b������˿ͨ�磻c��ͨ��������d��װ�������Լ�飻e������˿ֹͣͨ�硣
| ʵ��Ŀ�ģ����� ʵ��ԭ�������� ʵ��������ҩƷ������ ʵ��װ�ã����� ʵ�����ݴ��������� ʵ�������������� ʵ���������ۣ����� |
(3)Ϊȷ�ⶨ���ݣ�����Ϊ��װ���Ƿ�����?����Ҫ�Ľ�������ͼ����������ķ����ڻ����������ӵ�װ��ʾ��ͼ��ע����Ҫ���������ơ�������Ľ�����װ��ͼ�����߲��ָ�Ϊʵ�ߣ�
(4)ʵ�������ͬѧ������ʦ��ʵ�鱨����Ҫ��
Ŀ��ͼ(������������)���������ʵ�鱨���дҪ��
�Դ˷ݱ����������ۣ�������������������д��������������������Ŀո���д����ȱ��Ŀ ��
(5)��ʦ����ʵ�鱨���ָ�����ı�ʵ��ԭ��������
�Ƴ����Ӽ���ʵ�鷽�������û�ѧ����ʽ��ʾ�����
���·����ķ�Ӧԭ�� ���÷�����ⶨ������ ��