��Ŀ����
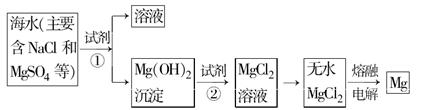
������һ���ḻ����Դ���⣬ͨ����ˮ���ۺ����ÿɻ���������ʹ�����ʹ�á�
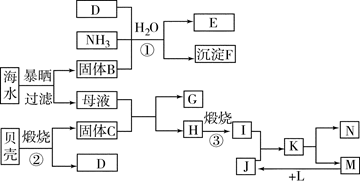
��1����ˮ���εĿ������ã�
�ٺ�ˮ����Ŀǰ�����Ϊ�������������ѡ��Զ�뽭���˺��ڣ�������꣬��ϫ��������ƽ̹�տ��ĺ�̲�����������Ϊ��ˮ�ء������غ� �ء�
��Ŀǰ��ҵ�ϲ��ñȽ��Ƚ������ӽ���Ĥ���۷������ȼҵ�������ڵ����������ӽ���Ĥֻ����������ͨ������ֹ�����Ӻ�����ͨ������˵���ȼ������������ӽ���Ĥ�����ã�
��дһ�㼴�ɣ���
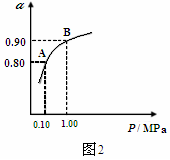
��2�����������ǽ�������չ������һ�ֽϺõĺ�ˮ������������ԭ����ͼ��ʾ�����о���ѡ���Ե������ӽ���Ĥ�������ӽ���Ĥ������С���ش���������⣺

�ٺ�ˮ����ֱ��ͨ�˵��������У������� ��
��A���ų����� �����ˮ����Ũˮ������
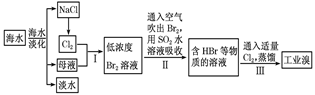
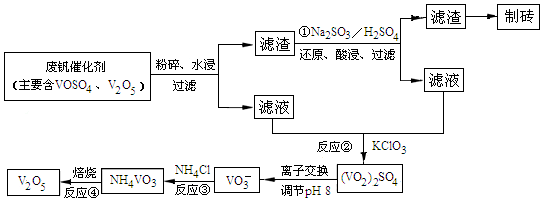
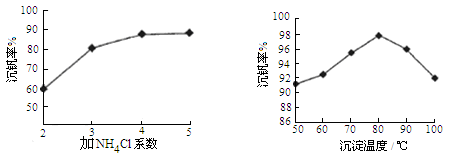
��3���ÿ�±����Na+��K+��Mg2+��Cl-��Br-�����ӣ�����ȡ�壬�������������£�
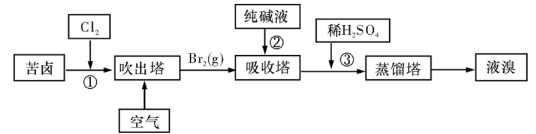
�����������е���Һ��Br03-�����������з�Ӧ�����ӷ���ʽΪ�� ��
��ͨ�����Ȼ��ѻ�ú�Br2����Һ��Ϊ�λ��辭�����������ա��ữ�����»�ú�Br2����Һ��
������������ͨ��ˮ�������ȣ������¶���90�����ҽ��������ԭ����
��1����ˮ���εĿ������ã�
�ٺ�ˮ����Ŀǰ�����Ϊ�������������ѡ��Զ�뽭���˺��ڣ�������꣬��ϫ��������ƽ̹�տ��ĺ�̲�����������Ϊ��ˮ�ء������غ� �ء�
��Ŀǰ��ҵ�ϲ��ñȽ��Ƚ������ӽ���Ĥ���۷������ȼҵ�������ڵ����������ӽ���Ĥֻ����������ͨ������ֹ�����Ӻ�����ͨ������˵���ȼ������������ӽ���Ĥ�����ã�
��дһ�㼴�ɣ���
��2�����������ǽ�������չ������һ�ֽϺõĺ�ˮ������������ԭ����ͼ��ʾ�����о���ѡ���Ե������ӽ���Ĥ�������ӽ���Ĥ������С���ش���������⣺

�ٺ�ˮ����ֱ��ͨ�˵��������У������� ��
��A���ų����� �����ˮ����Ũˮ������
��3���ÿ�±����Na+��K+��Mg2+��Cl-��Br-�����ӣ�����ȡ�壬�������������£�

�����������е���Һ��Br03-�����������з�Ӧ�����ӷ���ʽΪ�� ��
��ͨ�����Ȼ��ѻ�ú�Br2����Һ��Ϊ�λ��辭�����������ա��ữ�����»�ú�Br2����Һ��
������������ͨ��ˮ�������ȣ������¶���90�����ҽ��������ԭ����
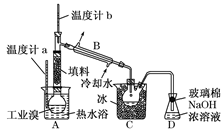
��1���ٽᾧ����ֹ����������������Ӧ����������ը����ֹ���������ɵ�����������Һ��Ӧ��ʹ�ռ��Ʒ����
��2���ٺ�ˮ�к��н϶��Ca2+��Mg2+�������ӣ����ʱ�����Mg(OH)2��Ca (OH)2�ȳ����Ӷ����������ӽ���Ĥ ��3�֣��ڵ�ˮ
��3����3CO32-+3Br2=5Br-+BrO3-+3CO2��
�ڸ����壬������Ũ��
���¶ȹ������Խ���������������¶ȹ����ֻὫ������ˮ������
��2���ٺ�ˮ�к��н϶��Ca2+��Mg2+�������ӣ����ʱ�����Mg(OH)2��Ca (OH)2�ȳ����Ӷ����������ӽ���Ĥ ��3�֣��ڵ�ˮ
��3����3CO32-+3Br2=5Br-+BrO3-+3CO2��
�ڸ����壬������Ũ��
���¶ȹ������Խ���������������¶ȹ����ֻὫ������ˮ������
�����������1���ٺ�ˮ�����е���������������Ϊ��ˮ�ء������غͽᾧ�أ�
�ڵ����������ӽ���Ĥֻ����������ͨ������ֹ�����Ӻ�����ͨ���������������������Ӵ���������Ӧ������ը��ͬʱҲ���������������ɵ�����������Һ��Ӧ��ʹ�ռ��Ʒ������
��2���١���ˮ�к��н϶��Ca2+��Mg2+�������ӣ����ʱ������������������Ũ�������뺣ˮ�е�Ca2+��Mg2+�������ӽ�ϻ����Mg(OH)2��Ca(OH)2�ȳ����Ӷ����������ӽ���Ĥ�����Ժ�ˮ����ֱ��ͨ�˵��������У�
�ڡ�A���ų�����ͼ�Тٴ���ˮ��װ���е����������������������������������������ӽ���Ĥֻ����������ͨ���������ӽ���Ĥֻ����������ͨ�������¢ٴ�����Ũ�ȼ�С���ڴ�����Ũ����������A�ڳ������ǵ�ˮ��
��3�������������е���Һ��Br03-��˵������̼������Һ����������ԭ��Ӧ���屻����ΪBr03-��ͬʱ�ֱ���ԭΪBr-�����ӷ���ʽΪ3CO32-+3Br2=5Br-+BrO3-+3CO2��
��ͨ�����Ȼ���ú�Br2����Һ����ĺ������٣��������������ա��ữ�����»�ú�Br2����Һ��Ŀ���Ǹ����壬������Ũ�ȡ�

��ϰ��ϵ�д�
 ��������������������ϵ�д�
��������������������ϵ�д�
�����Ŀ