��Ŀ����
��13�֣���������Ԫ��X��Y��Z��W��Q��ԭ��������������Yԭ�������������Ǵ�����2����X��W�ɷֱ���Z��ɳ���������X2Z��X2Z2��W2Z��W2Z2��Q�ĵ����dz����İ뵼����ϡ���ش�
��1��Y��Ԫ�����ڱ��е�λ���������������������� ��
��2��X2Z�ĵ���ʽ���������������������� ��
��3��W2Z2��X2Z��Ӧ�Ļ�ѧ����ʽ���������������������� ��
��4������Ԫ���������ַǽ���Ԫ��λ��ͬһ���塣��֤�����Ƿǽ�����ǿ����һ����Ӧ�Ļ�ѧ����ʽ���������������������� ��
��5���� 25�桢101 kPa�£�Q����̬�⻯������������ȫȼ�տɵ�Q������������Ӧ��ÿת��1mol e������190��0 kJ����÷�Ӧ���Ȼ�ѧ����ʽ���������������������� ��
��1��Y��Ԫ�����ڱ��е�λ���������������������� ��
��2��X2Z�ĵ���ʽ���������������������� ��
��3��W2Z2��X2Z��Ӧ�Ļ�ѧ����ʽ���������������������� ��
��4������Ԫ���������ַǽ���Ԫ��λ��ͬһ���塣��֤�����Ƿǽ�����ǿ����һ����Ӧ�Ļ�ѧ����ʽ���������������������� ��
��5���� 25�桢101 kPa�£�Q����̬�⻯������������ȫȼ�տɵ�Q������������Ӧ��ÿת��1mol e������190��0 kJ����÷�Ӧ���Ȼ�ѧ����ʽ���������������������� ��
(1)��2���ڢ�A��
(2)
(3)2Na202+2H20��4NaOH+O2
(4)CO2+H20+Na2SiO3��Na2CO3+H2Si03(����)
(5)SiH4(g)+202(g)��Si02(s)+2H2O(l)����AH=һ1520.0��kJ��mol
(2)

(3)2Na202+2H20��4NaOH+O2
(4)CO2+H20+Na2SiO3��Na2CO3+H2Si03(����)
(5)SiH4(g)+202(g)��Si02(s)+2H2O(l)����AH=һ1520.0��kJ��mol
Yԭ�������������Ǵ�����2������ȷ��YԪ����C��Q�ĵ����dz����İ뵼�������QΪSi��X��W�ɷֱ���Z��ɳ���������X2Z��X2Z2��W2Z��W2Z2�������Ĵ��������H2O��H2O2��Na2O,Na2O2�����ж�X��H��Z��O��W��Na;
��1�� C�����ڱ��е�λ���ǵ�2���ڢ�A��
��2�� X2Z��H2O������ʽ��
 ��3������������ˮ��Ӧ�ķ���ʽΪ2Na202+2H20��4NaOH+O2
��3������������ˮ��Ӧ�ķ���ʽΪ2Na202+2H20��4NaOH+O2
��4��̼Ԫ�غ�Ԫ��λ��ͬһ���壬֤�����ߵķǽ�����ǿ������ͨ������������Ӧˮ��������Դ�С���бȽϣ�CO2+H20+Na2SiO3��Na2CO3+H2Si03(����)��̼������Աȹ���ǿ��˵��̼�ķǽ����Աȹ�ǿ��
��5��Q����̬�⻯����SiH4����������ȼ��ʱת��1mol e������190��0 kJ����Եõ���1mol SiH4���뷴Ӧʱ�ų�������Ϊ1520.0��kJ�������Ȼ�ѧ����ʽΪSiH4(g)+202(g)��Si02(s)+2H2O(l)����AH=һ1520.0��kJ��mol��
��1�� C�����ڱ��е�λ���ǵ�2���ڢ�A��
��2�� X2Z��H2O������ʽ��

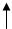 ��3������������ˮ��Ӧ�ķ���ʽΪ2Na202+2H20��4NaOH+O2
��3������������ˮ��Ӧ�ķ���ʽΪ2Na202+2H20��4NaOH+O2��4��̼Ԫ�غ�Ԫ��λ��ͬһ���壬֤�����ߵķǽ�����ǿ������ͨ������������Ӧˮ��������Դ�С���бȽϣ�CO2+H20+Na2SiO3��Na2CO3+H2Si03(����)��̼������Աȹ���ǿ��˵��̼�ķǽ����Աȹ�ǿ��
��5��Q����̬�⻯����SiH4����������ȼ��ʱת��1mol e������190��0 kJ����Եõ���1mol SiH4���뷴Ӧʱ�ų�������Ϊ1520.0��kJ�������Ȼ�ѧ����ʽΪSiH4(g)+202(g)��Si02(s)+2H2O(l)����AH=һ1520.0��kJ��mol��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 �Ժ�ĸ���Ʒ������Ҫ�ɷ��Ǿ��е�ˮƽ�����Ե�
�Ժ�ĸ���Ʒ������Ҫ�ɷ��Ǿ��е�ˮƽ�����Ե� �����й���
�����й��� ��й©�������й�
��й©�������й�