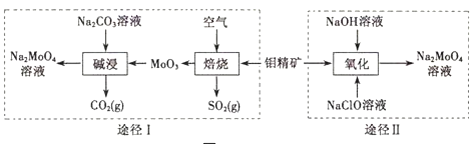
��Ŀ����
����Ŀ��úȼ���ŷŵ�������![]() ��Ҫ��
��Ҫ��![]() ��
��![]() ��
��![]() �γ����ꡢ��Ⱦ�������������������ش��������⣺
�γ����ꡢ��Ⱦ�������������������ش��������⣺

(1)����![]() ��������ɵõ��Ϻõ�Ч������֪���з�Ӧ��
��������ɵõ��Ϻõ�Ч������֪���з�Ӧ��
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
��Ӧ![]()
![]()
![]()
![]() ��
��![]() ______ ��
______ ��
(2)���ð�ˮ�����������տɵõ����ʣ�����ͬ���ʵ�����![]() ��
��![]() ����ˮ������Һ��
����ˮ������Һ��![]() ______
______ ![]() ����ĸ���
����ĸ���![]() ��
��
A.![]() /span>
/span>![]()
C.![]()
![]()
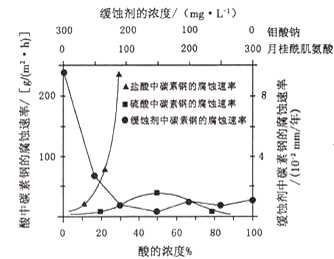
(3)�����ڽϸ��¶Ⱦ���ͼ1�����ѳ�![]() �����Ƶ�
�����Ƶ�![]() ��
��
���������ŵ�������� ______ ��
������������![]() �ĵ缫��Ӧʽ�� ______ ��
�ĵ缫��Ӧʽ�� ______ ��
����֪�����£�![]() ���ѳ�
���ѳ�![]() ���Ƶõ�
���Ƶõ�![]() ���
���![]() ��
��![]() ��Һ����
��Һ����![]() ��
��![]() ��Һ��ϣ������û����Һ��
��Һ��ϣ������û����Һ��![]() ,��
,��![]() ��Һ��
��Һ��![]() ��Һ�������Ϊ ______
��Һ�������Ϊ ______ ![]() ��ʹ��Һ��
��ʹ��Һ��![]() ����Ӧ������Һ��
����Ӧ������Һ��![]() ______
______ ![]() ��
��
(4)һ�������£���![]() ��NiO��
��NiO��![]() ���������������·�Ӧ����ȼú�����е���ӦΪ��
���������������·�Ӧ����ȼú�����е���ӦΪ��![]()
![]() ����������ͬ��������ͬʱ��
����������ͬ��������ͬʱ��![]() ��ת�����淴Ӧ�¶ȵı仯��ͼ2�������Ǵ����ļ۸����أ�ѡ�� ______ Ϊ�÷�Ӧ�Ĵ�����Ϊ����
��ת�����淴Ӧ�¶ȵı仯��ͼ2�������Ǵ����ļ۸����أ�ѡ�� ______ Ϊ�÷�Ӧ�Ĵ�����Ϊ����![]() ѡ�����
ѡ�����![]() ��
��
![]()
![]()
![]()
ѡ��ô����������ǣ� ______ ��
ij����С����ѡ��Ĵ�������![]() ʱ���о���
ʱ���о���![]() ��
��![]() �ֱ�Ϊ1��1��3��1ʱ��
�ֱ�Ϊ1��1��3��1ʱ��![]() ת���ʵı仯���
ת���ʵı仯���![]() ͼ
ͼ![]() ��ͼ3�б�ʾ
��ͼ3�б�ʾ![]() ��
��![]() ��1�ı仯����Ϊ ______ ��
��1�ı仯����Ϊ ______ ��
���𰸡�![]() BD
BD ![]()
![]() 10��1
10��1 ![]() c
c ![]() ������ʱ������Խϵ��¶ȿɻ�ýϸߵ�
������ʱ������Խϵ��¶ȿɻ�ýϸߵ�![]() ת���ʣ��Ӷ���Լ��Դ a
ת���ʣ��Ӷ���Լ��Դ a
��������
��1�����ݸ�˹���ɿ�֪��+��-�ۼ��õ���Ӧ��
��2����ͬ���ʵ�����SO2��NH3����ˮ��Ӧ������������泥������Һ�е���غ�������غ�����ж�ѡ�
��3��������������ԭ��Ӧ����������������Ӧ����ʾ��ͼ��֪��������������õ��ӵõ�SO42-��
������������������������ʧȥ��������SO3��O2��
��ˮ�����ӻ�����KW=10-14��pH=9��Ba��OH��2��Һ������������Ũ��Ϊ0.00001mol/L��pH=4��H2SO4��Һ��������Ũ��Ϊ0.0001mol/L�������û��ҺΪ���ԣ����ǡ����ȫ��Ӧ�������ӵ����ʵ����������������ӵ����ʵ������ݴ���ʽ�����Ba��OH��2 ��Һ�� H2SO4 ��Һ������ȵ�ֵ������Q=c��Ba2+��c��SO42-��������Һ��c��SO42-����1.0��10-5molL-1����Ӧ������Һ�� c��Ba2+����ֵ��
��4��Fe2O3������ʱ������Խϵ��¶ȿɻ�ýϸߵ�SO2ת���ʣ��Ӷ���Լ��Դ�� n��CO����n��SO2��Խ���������ת����Խ��
��1����֪![]()
![]()
![]()
����ݸ�˹���ɿ�֪![]() ���õ���Ӧ
���õ���Ӧ![]() ��
��
�ʴ�Ϊ��![]() ��
��
��2����ͬ���ʵ�����![]() ��
��![]() ����ˮ������Ӧ������������泥���Ӧ�����ӷ���ʽΪ��
����ˮ������Ӧ������������泥���Ӧ�����ӷ���ʽΪ��![]() ����Һ�д��ڵ���غ㣺
����Һ�д��ڵ���غ㣺![]() ��
��![]() ��D��ȷ��C������Һ�д��������غ㣺
��D��ȷ��C������Һ�д��������غ㣺![]() ����ϵ���غ�������غ��֪
����ϵ���غ�������غ��֪![]() ������A����B��ȷ��
������A����B��ȷ��
�ʴ�Ϊ��BD��
��3������������ԭ��Ӧ����������������Ӧ����ʾ��ͼ��֪��������������õ��ӵõ�![]() ������������������������ʧȥ��������
������������������������ʧȥ��������![]() ��
��![]() ��
��
��������������õ��ӣ��ʴ�Ϊ��![]() ��
��
�������缫��Ӧ����ʽΪ��![]() ��
��
�ʴ�Ϊ��![]() ��
��
�������£�ˮ�����ӻ�����![]() ��
��![]() ��
��![]() ��Һ������������Ũ��Ϊ
��Һ������������Ũ��Ϊ![]() ��
��![]() ��
��![]() ��Һ��������Ũ��Ϊ
��Һ��������Ũ��Ϊ![]() �������û��ҺΪ���ԣ����ǡ����ȫ��Ӧ�����У�
�������û��ҺΪ���ԣ����ǡ����ȫ��Ӧ�����У�![]() ����
����![]() ��Һ��
��Һ��![]() ��Һ������ֱ�ΪaL��bL������
��Һ������ֱ�ΪaL��bL������![]() ��a��
��a��![]() ��1��
��1��![]() �������£�
�������£�![]() ����ʹ��Һ��
����ʹ��Һ��![]() �����
�����![]() ��֪��Һ��Ӧ����
��֪��Һ��Ӧ����![]() ��
��
�ʴ�Ϊ��10��1�� ![]() ��
��
��4��Fe2O3������ʱ������Խϵ��¶ȿɻ�ýϸߵ�![]() ת���ʣ��Ӷ���Լ��Դ����ѡFe2O3��������
ת���ʣ��Ӷ���Լ��Դ����ѡFe2O3��������![]() ��
��![]() Խ���������ת����Խ������a��ʾ
Խ���������ת����Խ������a��ʾ![]() ��
��![]() ��1�ı仯���ߣ�
��1�ı仯���ߣ�
�ʴ�Ϊ��c��![]() ������ʱ������Խϵ��¶ȿɻ�ýϸߵ�
������ʱ������Խϵ��¶ȿɻ�ýϸߵ�![]() ת���ʣ��Ӷ���Լ��Դ��a��
ת���ʣ��Ӷ���Լ��Դ��a��

 ��У����ϵ�д�
��У����ϵ�д�