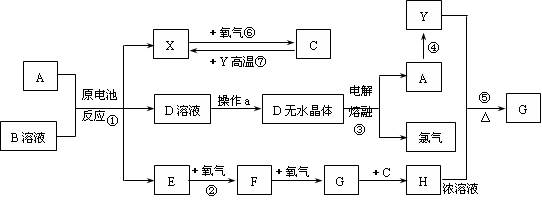
��Ŀ����
��ҵ����ȡ����ʯ(Na3AlF6)�Ļ�ѧ����ʽ���£�
2Al(OH)3��12HF��3Na2CO3��2Na3AlF6��3CO2����9H2O�������������������գ�
����������Ӧ�ķ�Ӧ����������У�CO2�ĵ���ʽ ����������ĵ��뷽��ʽ ����0.1mol?L��1�ĸ�������Һ��pHΪa���������ĵ����Ϊ ����a��ʾ��
�Ʒ�Ӧ����������Ԫ����Ԫ�����ڱ���λ�����ڣ��������ж����ǵĽ����Ի�ǽ�����ǿ������ ��ѡ���ţ���
a����̬�⻯����ȶ��� b������������Ӧˮ���������
c��������������Ӧ������ d��������ͬŨ���ᷢ����Ӧ�Ŀ���
�Ƿ�Ӧ����ijЩԪ�ش���ͬһ���ڡ���������������Ӧ��ˮ����֮�䷢����Ӧ�����ӷ���ʽΪ ��
��Na2CO3�׳ƴ������ ���塣��ҵ����ȡ�����ԭ���� ��
��F2������NaOH��Һ������OF2��д���÷�Ӧ�Ļ�ѧ����ʽ����ƽ
2Al(OH)3��12HF��3Na2CO3��2Na3AlF6��3CO2����9H2O�������������������գ�
����������Ӧ�ķ�Ӧ����������У�CO2�ĵ���ʽ ����������ĵ��뷽��ʽ ����0.1mol?L��1�ĸ�������Һ��pHΪa���������ĵ����Ϊ ����a��ʾ��
�Ʒ�Ӧ����������Ԫ����Ԫ�����ڱ���λ�����ڣ��������ж����ǵĽ����Ի�ǽ�����ǿ������ ��ѡ���ţ���
a����̬�⻯����ȶ��� b������������Ӧˮ���������
c��������������Ӧ������ d��������ͬŨ���ᷢ����Ӧ�Ŀ���
�Ƿ�Ӧ����ijЩԪ�ش���ͬһ���ڡ���������������Ӧ��ˮ����֮�䷢����Ӧ�����ӷ���ʽΪ ��
��Na2CO3�׳ƴ������ ���塣��ҵ����ȡ�����ԭ���� ��
��F2������NaOH��Һ������OF2��д���÷�Ӧ�Ļ�ѧ����ʽ����ƽ
��1��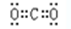 ��HF
��HF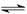 H++F- 10a+1��100% ��2��ac
H++F- 10a+1��100% ��2��ac
��3��Al(OH)3+OH-=AlO2-+2H2O
������ NH3��NaCl������Һ��CO2��
��2F2��2NaOH��2NaF��OF2��H2O
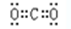 ��HF
��HF H++F- 10a+1��100% ��2��ac
H++F- 10a+1��100% ��2��ac��3��Al(OH)3+OH-=AlO2-+2H2O
������ NH3��NaCl������Һ��CO2��
��2F2��2NaOH��2NaF��OF2��H2O
��1��CO2�Ǻ��м��Լ��Ĺ��ۻ��������ʽΪ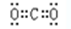 ��HF��������ڵ���ƽ�⣬����ʽΪHF
��HF��������ڵ���ƽ�⣬����ʽΪHF H++F-��pH��a����������Ũ����10��amol/L�����Ե����
H++F-��pH��a����������Ũ����10��amol/L�����Ե����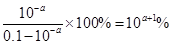
��2������Ԫ�طֱ���O��F���ǽ����ԵıȽϹ��ɣ�
1����Ԫ��ԭ�ӵ��������жϣ�һ������£�������Խǿ����Ӧ�ǽ�����Խǿ��
2���ɵ��ʺ�����ߺ�ˮ�ķ�Ӧ�̶��жϣ���ӦԽ���ң��ǽ�����Խǿ��
3���ɶ�Ӧ�⻯����ȶ����жϣ��⻯��Խ�ȶ����ǽ�����Խǿ��
4���ɺ��������ϵ����׳̶��жϣ�����Խ���ף��ǽ�����Խǿ��
5��������������Ӧˮ������������жϣ�����Խǿ���ǽ���Խǿ��������Ԫ��֮�⣩
6���ɶ�Ӧ�����ӵĻ�ԭ���жϣ���ԭ��Խǿ����Ӧ�ǽ�����Խ����
7�����û���Ӧ�жϣ�ǿ���������������û���Ӧ��˵��Ԫ�صķǽ�����ǿ������ǽ�������Ӧ�����������ǽ�����������ԭ�����û���Ӧ������Ϊ�ȽϷǽ�����ǿ�������ݡ�
ֵ��ע����ǣ���Ԫ��û������̬����û�з��ĺ����ᣬ��������������Ӧˮ�����������ǿ���Ǹ����ᣬ�����Ƿǽ����Ը����ȵķ�Ԫ�أ��ʹ���5ֻ�����ڷ�Ԫ��֮��ķǽ���Ԫ�ء�
8����Ԫ�������ɣ�ͬ����Ԫ�������ң���˵���������ӣ��ǽ�������ǿ��ͬ����Ԫ�����ϵ��£���˵���������ӣ��ǽ����Լ�����
�ݴ˿�֪ѡ��ac��ȷ����ѡac��
��3������������������������ܺ��������Ʒ�Ӧ������ʽΪAl(OH)3+OH-=AlO2-+2H2O��
��4��̼���������ӻ�����γɵľ��������Ӿ��壬��ȡ̼���Ƶ�ԭ����NH3��NaCl������Һ��CO2��
��5������ԭ���غ��֪��������������Ƿ����ƺ�ˮ������ʽΪ2F2��2NaOH��2NaF��OF2��H2O��
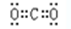 ��HF��������ڵ���ƽ�⣬����ʽΪHF
��HF��������ڵ���ƽ�⣬����ʽΪHF H++F-��pH��a����������Ũ����10��amol/L�����Ե����
H++F-��pH��a����������Ũ����10��amol/L�����Ե����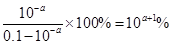
��2������Ԫ�طֱ���O��F���ǽ����ԵıȽϹ��ɣ�
1����Ԫ��ԭ�ӵ��������жϣ�һ������£�������Խǿ����Ӧ�ǽ�����Խǿ��
2���ɵ��ʺ�����ߺ�ˮ�ķ�Ӧ�̶��жϣ���ӦԽ���ң��ǽ�����Խǿ��
3���ɶ�Ӧ�⻯����ȶ����жϣ��⻯��Խ�ȶ����ǽ�����Խǿ��
4���ɺ��������ϵ����׳̶��жϣ�����Խ���ף��ǽ�����Խǿ��
5��������������Ӧˮ������������жϣ�����Խǿ���ǽ���Խǿ��������Ԫ��֮�⣩
6���ɶ�Ӧ�����ӵĻ�ԭ���жϣ���ԭ��Խǿ����Ӧ�ǽ�����Խ����
7�����û���Ӧ�жϣ�ǿ���������������û���Ӧ��˵��Ԫ�صķǽ�����ǿ������ǽ�������Ӧ�����������ǽ�����������ԭ�����û���Ӧ������Ϊ�ȽϷǽ�����ǿ�������ݡ�
ֵ��ע����ǣ���Ԫ��û������̬����û�з��ĺ����ᣬ��������������Ӧˮ�����������ǿ���Ǹ����ᣬ�����Ƿǽ����Ը����ȵķ�Ԫ�أ��ʹ���5ֻ�����ڷ�Ԫ��֮��ķǽ���Ԫ�ء�
8����Ԫ�������ɣ�ͬ����Ԫ�������ң���˵���������ӣ��ǽ�������ǿ��ͬ����Ԫ�����ϵ��£���˵���������ӣ��ǽ����Լ�����
�ݴ˿�֪ѡ��ac��ȷ����ѡac��
��3������������������������ܺ��������Ʒ�Ӧ������ʽΪAl(OH)3+OH-=AlO2-+2H2O��
��4��̼���������ӻ�����γɵľ��������Ӿ��壬��ȡ̼���Ƶ�ԭ����NH3��NaCl������Һ��CO2��
��5������ԭ���غ��֪��������������Ƿ����ƺ�ˮ������ʽΪ2F2��2NaOH��2NaF��OF2��H2O��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
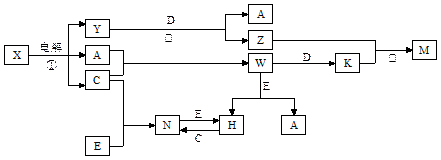
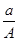 (A��N��m)mol
(A��N��m)mol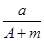 (A��N)mol
(A��N)mol