��Ŀ����
2009��12��7��һ18���ڵ������籾�����ٿ������Ϲ�������飬��δ��Ӧ������仯��ȫ���ж�ǩ���µ�Э�飮����ν��ʹ�����CO2�ĺ�������Ч�ؿ�������CO2��������ȫ������ձ����ӣ�Ϊ��С������CO2�Ի�����Ӱ�죬һ����������������������ŷ�������һ�����ѧ�Ҽ�ǿ�˶�CO2�������õ��о�����1��Ŀǰ��ҵ����һ�ַ�������CO2������ȼ�ϼ״���Ϊ̽���÷�Ӧԭ������������ʵ�飺
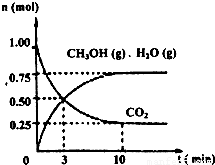
ij�¶��£����ݻ�Ϊ2L���ܱ������У�����1mol CO2��3.25mol H2����һ�������·�����Ӧ�����CO2��CH3OH��g����H2O��g�������ʵ�����n����ʱ��仯����ͼ��ʾ��
�ٴӷ�Ӧ��ʼ��ƽ�⣬������ƽ����Ӧ����v��H2��=______��
�����д�ʩ��һ������ʹn��CH3OH��/n��CO2��������ǣ�______��
A�������¶� B����С�������ݻ� C����ˮ��������ϵ�з��� D��ʹ�ø���Ч�Ĵ���
��2�����³�ѹ�£�����CO 2ˮ��Һ��pH=5.6��c��H2CO3��=1.5×l0-5mol?L-1��������ˮ�ĵ��뼰H2CO3�ĵڶ������룬��H2CO3
 HCO3-+H+�ĵ���ƽ�ⳣ��K=______������֪��10 -5.6=2.5×l0-6����
HCO3-+H+�ĵ���ƽ�ⳣ��K=______������֪��10 -5.6=2.5×l0-6������3����״���£���1.12LCO2ͨ��100mL 1mol?L-1��NaOH��Һ�У�������Һ������Ũ���ɴ�С��˳��Ϊ______��
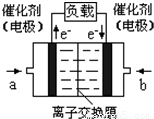
��4����ͼ���Ҵ�ȼ�ϵ�أ��������ҺΪKOH��Һ���Ľṹʾ��ͼ����a��ͨ�����______����Ҵ���������������
b���缫�Ϸ����ĵ缫��Ӧ�ǣ�______��
��5��CO2����Ȼ��ѭ��ʱ����CaCO3��Ӧ��CaCO3��һ���������ʣ���Ksp=2.8×10-9��CaCl2��Һ��Na2CO3��Һ��Ͽ��γ�CaCO3�������ֽ��������CaCl2��Һ��Na2CO3��Һ��ϣ���Na2CO3��Һ��Ũ��Ϊ2×10-4mo1/L�������ɳ�������CaCl2��Һ����СŨ��Ϊ______��
���𰸡���������1���ٷ�Ӧ�ķ���ʽΪCO2+3H2�TCH3OH+H2O�����ݷ�Ӧ����֮�ȵ��ڻ�ѧ������֮�ȼ��������ķ�Ӧ���ʣ�
�ڸ���ƽ���ƶ����������
��2������ƽ�ⳣ��K��H2CO3��=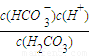 ���㣻
���㣻
��3��1.12LCO2ͨ��100mL1mol/L��NaOH��Һ��Ӧ������0.05molNa2CO3��CO32-ˮ��ˮ�⣬��Һ�ʼ��ԣ��Դ��ж�����Ũ�ȴ�С��
��4���ڼ��Ե������Һ��ͨ���Ҵ����Ҵ�ӦΪԭ��صĸ���������������Ӧ���Ҵ�������ΪCO32-���ӣ�����������ͨ�룬����ԭΪOH-��������ӦΪO2+4e-+2H2O=4OH-��
��5�������ܶȻ���������Ca2+���ӵ�Ũ�ȣ�
����⣺��1���ٷ�Ӧ�ķ���ʽΪCO2+3H2�TCH3OH+H2O����Ӧ��v��H2��=3v��CO2��= ×3=0.1125mol/��L?min����
×3=0.1125mol/��L?min����
�ʴ�Ϊ��0.1125mol/��L?min����
��A���÷�Ӧ�ķ�Ӧ��δ֪���������жϣ���A����
B����С�������ݻ�����ѹǿ����ƽ���������С�ķ����ƶ�����������Ӧ�����ƶ���n��CH3OH������n��CO2����С��n��CH3OH��/n��CO2������B����
C����ˮ��������ϵ�з��룬ƽ��������Ӧ�����ƶ���n��CH3OH������n��CO2����С��n��CH3OH��/n��CO2������C����
D��ʹ�ø���Ч�Ĵ��������̵���ƽ���ʱ�䣬ƽ�ⲻ�ƶ���n��CH3OH��/n��CO2�����䣬��D��ȷ��
�ʴ�Ϊ��D��
��2��K��H2CO3��=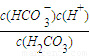 =
=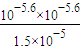 =4.2×10-7���ʴ�Ϊ��4.2×10-7��
=4.2×10-7���ʴ�Ϊ��4.2×10-7��
��3��1.12LCO2ͨ��100mL1mol/L��NaOH��Һ��Ӧ������0.05molNa2CO3��CO32-ˮ�⣬��Һ�ʼ��ԣ���Һ�ʼ��ԣ���c��OH-����c��H+����
��ˮ��̶Ƚ�С����c��CO32-����c��OH-����ˮ��OH-��Դ��CO32-ˮ�⼰ˮ�ĵ��룬��OH-����c��HCO3-����ˮ�ĵ���̶Ⱥ�Сc��HCO3-����c��H+����
��Ϊc��Na+����c��CO32-����c��OH-����c��HCO3-����c��H+����
�ʴ�Ϊ��c��Na+����c��CO32-����c��OH-����c��HCO3-����c��H+����
��4���ڼ��Ե������Һ��ͨ���Ҵ����Ҵ�ӦΪԭ��صĸ���������������Ӧ���Ҵ�������ΪCO32-���ӣ�����������ͨ�룬����ԭΪOH-��������ӦΪO2+4e-+2H2O=4OH-��
�ʴ�Ϊ���Ҵ���O2+4e-+2H2O=4OH-��
��5���������CaCl2��Һ��Na2CO3��Һ���ʱ��̼������Һ��Ũ�ȱ�Ϊ1×10-4mo1/L�������ܶȻ��������㣬c��Ca2+��=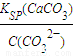 =
=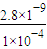 =2.8×10-5mo1/L����Ϊ�ǵ������ϣ����Ի��ǰ��Һ��Ũ���ǻ�Ϻ��2�������Ի��ǰ��Һ��Ũ��Ϊ2.8×10-5mo1/L×2=5.6×10-5mo1/L��
=2.8×10-5mo1/L����Ϊ�ǵ������ϣ����Ի��ǰ��Һ��Ũ���ǻ�Ϻ��2�������Ի��ǰ��Һ��Ũ��Ϊ2.8×10-5mo1/L×2=5.6×10-5mo1/L��
�ʴ�Ϊ��5.6×10-5mo1/L��
���������⿼���Ϊ�ۺϣ��漰��Ӧ���ʡ���ѧƽ�⡢�����ˮ�⡢ԭ����Լ����ܵ���ʵ��ܽ�ƽ��ĵ�֪ʶ����Ŀ�ѶȽϴ����״���Ϊƽ���ƶ�������Ũ�ȴ�С�ıȽ��������⣬����ʱע���������ķ�����
�ڸ���ƽ���ƶ����������
��2������ƽ�ⳣ��K��H2CO3��=
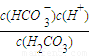 ���㣻
���㣻��3��1.12LCO2ͨ��100mL1mol/L��NaOH��Һ��Ӧ������0.05molNa2CO3��CO32-ˮ��ˮ�⣬��Һ�ʼ��ԣ��Դ��ж�����Ũ�ȴ�С��
��4���ڼ��Ե������Һ��ͨ���Ҵ����Ҵ�ӦΪԭ��صĸ���������������Ӧ���Ҵ�������ΪCO32-���ӣ�����������ͨ�룬����ԭΪOH-��������ӦΪO2+4e-+2H2O=4OH-��
��5�������ܶȻ���������Ca2+���ӵ�Ũ�ȣ�
����⣺��1���ٷ�Ӧ�ķ���ʽΪCO2+3H2�TCH3OH+H2O����Ӧ��v��H2��=3v��CO2��=
 ×3=0.1125mol/��L?min����
×3=0.1125mol/��L?min�����ʴ�Ϊ��0.1125mol/��L?min����
��A���÷�Ӧ�ķ�Ӧ��δ֪���������жϣ���A����
B����С�������ݻ�����ѹǿ����ƽ���������С�ķ����ƶ�����������Ӧ�����ƶ���n��CH3OH������n��CO2����С��n��CH3OH��/n��CO2������B����
C����ˮ��������ϵ�з��룬ƽ��������Ӧ�����ƶ���n��CH3OH������n��CO2����С��n��CH3OH��/n��CO2������C����
D��ʹ�ø���Ч�Ĵ��������̵���ƽ���ʱ�䣬ƽ�ⲻ�ƶ���n��CH3OH��/n��CO2�����䣬��D��ȷ��
�ʴ�Ϊ��D��
��2��K��H2CO3��=
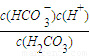 =
=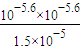 =4.2×10-7���ʴ�Ϊ��4.2×10-7��
=4.2×10-7���ʴ�Ϊ��4.2×10-7����3��1.12LCO2ͨ��100mL1mol/L��NaOH��Һ��Ӧ������0.05molNa2CO3��CO32-ˮ�⣬��Һ�ʼ��ԣ���Һ�ʼ��ԣ���c��OH-����c��H+����
��ˮ��̶Ƚ�С����c��CO32-����c��OH-����ˮ��OH-��Դ��CO32-ˮ�⼰ˮ�ĵ��룬��OH-����c��HCO3-����ˮ�ĵ���̶Ⱥ�Сc��HCO3-����c��H+����
��Ϊc��Na+����c��CO32-����c��OH-����c��HCO3-����c��H+����
�ʴ�Ϊ��c��Na+����c��CO32-����c��OH-����c��HCO3-����c��H+����
��4���ڼ��Ե������Һ��ͨ���Ҵ����Ҵ�ӦΪԭ��صĸ���������������Ӧ���Ҵ�������ΪCO32-���ӣ�����������ͨ�룬����ԭΪOH-��������ӦΪO2+4e-+2H2O=4OH-��
�ʴ�Ϊ���Ҵ���O2+4e-+2H2O=4OH-��
��5���������CaCl2��Һ��Na2CO3��Һ���ʱ��̼������Һ��Ũ�ȱ�Ϊ1×10-4mo1/L�������ܶȻ��������㣬c��Ca2+��=
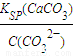 =
=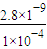 =2.8×10-5mo1/L����Ϊ�ǵ������ϣ����Ի��ǰ��Һ��Ũ���ǻ�Ϻ��2�������Ի��ǰ��Һ��Ũ��Ϊ2.8×10-5mo1/L×2=5.6×10-5mo1/L��
=2.8×10-5mo1/L����Ϊ�ǵ������ϣ����Ի��ǰ��Һ��Ũ���ǻ�Ϻ��2�������Ի��ǰ��Һ��Ũ��Ϊ2.8×10-5mo1/L×2=5.6×10-5mo1/L���ʴ�Ϊ��5.6×10-5mo1/L��
���������⿼���Ϊ�ۺϣ��漰��Ӧ���ʡ���ѧƽ�⡢�����ˮ�⡢ԭ����Լ����ܵ���ʵ��ܽ�ƽ��ĵ�֪ʶ����Ŀ�ѶȽϴ����״���Ϊƽ���ƶ�������Ũ�ȴ�С�ıȽ��������⣬����ʱע���������ķ�����

��ϰ��ϵ�д�
 Ӯ�ڿ�����ʦ��ʱ�ƻ�ϵ�д�
Ӯ�ڿ�����ʦ��ʱ�ƻ�ϵ�д�
�����Ŀ
2009��12��7�գ������Ϲ�����仯��ܹ�Լ����15�ε�Լ�������ڵ������籾�����ٿ���192�����Һ͵����Ĵ�����ϯ�˻��飮�˴λ��鱻��Ϊȫ�������϶���ȫ���ů�ж�һ�κ���ҪŬ����������ʹ�����ů�������ǣ�������
| A��NO2 | B��SO2 | C��H2S | D��CO2 |
2009��12��7���ڸ籾�����ٿ����������ϣ���Ҫ���ۼ���CO2���ŷ�������������̼���á��͡���̼��������в�������һ������ǣ�������
| A������һ������Ʒ��ʹ�� | B������̫��������ˮ���к�ˮ�ĵ��� | C�������Լݳ��У��ܿ�������ͨ��ӵ�� | D����ˮ�͵�ˮ���㴦��װ��Ĥ������ˮ�е����Ũ�ȵIJ�����з��� |
 LiFePO4����ص�����������LiFePO4������������ʯī���������Ϊ����ʣ��������-п��ط�Ӧ��3Zn+2K2FeO4+8H2O
LiFePO4����ص�����������LiFePO4������������ʯī���������Ϊ����ʣ��������-п��ط�Ӧ��3Zn+2K2FeO4+8H2O 3Zn��OH��2+2Fe��OH��3+4KOH
3Zn��OH��2+2Fe��OH��3+4KOH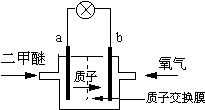 �籾��������仯�����2009��12��7�տ�Ļ�������ͱ����ԡ���ʷ��������Ҫ�Ļ��顱�����ı�������˵Ļ��顱�ȸ���������ͷ�Σ���Ȼ��������Ծͼ�����������ż��Ľ���������̼���������õ��˹㷺��ͬ���й��������¹��2010��11��5�Ŵ����ĵ�̼֮��--���������ٻص���ǰ���õ�̼�������뵽ÿ���˵�����֮�У���̼��ÿ���˵����Σ�����Դ�����е�ȼ�ϵ���ǵ�̼�о�����Ҫ����
�籾��������仯�����2009��12��7�տ�Ļ�������ͱ����ԡ���ʷ��������Ҫ�Ļ��顱�����ı�������˵Ļ��顱�ȸ���������ͷ�Σ���Ȼ��������Ծͼ�����������ż��Ľ���������̼���������õ��˹㷺��ͬ���й��������¹��2010��11��5�Ŵ����ĵ�̼֮��--���������ٻص���ǰ���õ�̼�������뵽ÿ���˵�����֮�У���̼��ÿ���˵����Σ�����Դ�����е�ȼ�ϵ���ǵ�̼�о�����Ҫ����