��Ŀ����
12����Ҫ�����������գ���1����֪�ڱ�״���µ��������� ��6.72L CH4 ��3.01��1023��HCl���Ӣ�13.6��H2S ��0.2molNH3
����Ӧ�������д���пհף�
��������Ǣڣ��ܶ������Ǣڣ�
������С���Ǣܣ�����ԭ���������Ǣ٣�
��2��0.6mol ��������0.4mol���� O3����֮��Ϊ1��1�����Ӹ���֮��Ϊ3��2����ԭ�Ӹ���֮��Ϊ1��1��
��3��ij�����Ȼ���MCl3 26.7g������0.600mol Cl-�������M�����ԭ������Ϊ27g/mol��
���� �����Vm=22.4L/mol������n=$\frac{V}{{V}_{m}}$=$\frac{N}{{N}_{A}}$=$\frac{m}{M}$����=$\frac{m}{V}$=$\frac{M}{{V}_{m}}$��Ϸ��ӵ���ɼ����й���������
��� �⣺��1����6.72LCH4�У�n��CH4��=$\frac{6.72L}{22.4L/mol}$=0.3mol��m��CH4��=0.3mol��16g/mol=4.8g���ѣ�CH4��=$\frac{m}{V}$=$\frac{M}{{V}_{m}}$=$\frac{16}{22.4}$g/L��
N��H��=4N��CH4��=1.2NA��
��3.01��1023��HCl������n��HCl��=$\frac{3.01��1{0}^{23}}{6.02{��10}^{23}}$mol=0.5mol��V��HCl��=0.5mol��22.4L/mol=11.2L��
�ѣ�HCl��=$\frac{m}{V}$=$\frac{M}{{V}_{m}}$=$\frac{36.5}{22.4}$g/L��m��HCl��=0.5mol��36.5g/mol=18.25g��
��13.6��H2S �У�n��H2S��=$\frac{13.6g}{34g/mol}$=0.4mol��V��H2S��=0.4mol��22.4L/mol=8.96L���ѣ�H2S��=$\frac{m}{V}$=$\frac{M}{{V}_{m}}$=$\frac{34}{22.4}$g/L��
N��H��=2N��H2S��=0.8NA��
��0.2molNH3�У�m��NH3��=0.2mol��17g/mol=3.4g��V��NH3��=0.2mol��22.4L/mol=4.48L���ѣ�NH3��=$\frac{m}{V}$=$\frac{M}{{V}_{m}}$=$\frac{17}{22.4}$g/L��N��H��=3N��NH3��=0.6NH3��
���ԣ���������Ǣڣ��ܶ������Ǣڣ�������С���Ǣܣ�����ԭ���������Ǣ٣�
�ʴ�Ϊ���ڣ��ڣ��ܣ��٣�
��2��0.6mol ��������0.4mol���� O3����֮��Ϊ0.4mol��32g/mol��0.4mol��48g/mol=1��1��
���Ӹ���֮��Ϊ0.6��0.4=3��2��
��ԭ�Ӹ���֮��Ϊ0.6mol��2��0.4mol��3=1��1��
�ʴ�Ϊ��1��1��3��2��1��1��
��3��ij�����Ȼ���MCl3 26.7g������0.600mol Cl-����n��MCl3��=0.200mol��M��MCl3��=$\frac{26.7g}{0.2mol}$=133.5g/mol��
M��M��=133.5g/mol-3��35.5g/mol=27g/mol��
�ʴ�Ϊ��27g/mol��
���� ���⿼�����ʵ�������ؼ��㣬Ϊ��Ƶ���㣬������ѧ���ķ��������������Ŀ��飬��Ŀ�ѶȲ���ע����ؼ��㹫ʽ�����ã�

 Ӯ�ڿ�����ʦ��ʱ�ƻ�ϵ�д�
Ӯ�ڿ�����ʦ��ʱ�ƻ�ϵ�д� �������Ͽ�ʱͬ��ѵ��ϵ�д�
�������Ͽ�ʱͬ��ѵ��ϵ�д� �������ͬ����ϰϵ�д�
�������ͬ����ϰϵ�д�| A�� | �Ƹ���ˮ���� | B�� | ���ۻ���С�� | ||
| C�� | ��Һ�в����϶������ | D�� | ˮ��Һ��ɺ�ɫ |
| A�� | ��ռ�����һ����� | B�� | ����ԭ��������� | ||
| C�� | ��������������� | D�� | ������������� |




 ��Dԭ�ӵ�ԭ�ӽṹʾ��ͼΪ
��Dԭ�ӵ�ԭ�ӽṹʾ��ͼΪ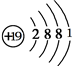 ��C+���ӵĽṹʾ��ͼΪ
��C+���ӵĽṹʾ��ͼΪ ��
��