��Ŀ����
(14��)�����⻯�����MH��Ni�������������ܡ���ȫ������Ⱦ������ЧӦ���۸����ˣ��ѳ�ΪĿǰ��߷�չǰ���ġ���ɫ��Դ�����֮һ������ܷ�ӦΪMH+NiOOH 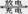 M+Ni(OH)2��MΪ����Ͻ�MHΪ��������ԭ�ӵĴ���Ͻ𡣵������ҺΪŨKOH��Һ��
M+Ni(OH)2��MΪ����Ͻ�MHΪ��������ԭ�ӵĴ���Ͻ𡣵������ҺΪŨKOH��Һ��
(1)д���ŵ�ʱ�ĸ�����Ӧ_________________
(2)���ʱ�������ĵ缫��ӦΪ__________________
����������������Ni(OH)2��̼�ۡ���������Ϳ�����������Ƴɡ�ij��ȤС��Ըõ�ص缫���Ͻ�����Դ�����о������ʵ���������£�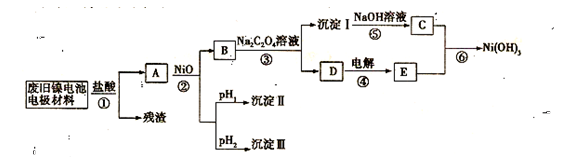
��֪����NiCl2������ˮ��Fe3+��������Ni2+
��ij�¶���һЩ�������������Ksp����������������pH���±���ʾ��
| M(OH)x | Ksp | pH | |
| ��ʼ���� | ������ȫ | ||
| Al(OH)3 | 1.9��10-23 | 3.43 | 4.19 |
| Fe(OH)3 | 3.8��10-38 | 2.53 | 2.94 |
| Ni(OH)2 | 1.6��10-14 | 7.60 | 9.75 |
�ش��������⣺
��3�������ϱ������жϲ�����������ij�����Ϊ ���������ij���Ϊ��Ϊ__________________���ѧʽ������pH1 pH2�����>������=����<������
��4����֪�ܽ�ȣ�NiC2O4 > NiC2O4��H2O > NiC2O4��2H2O����۵Ļ�ѧ����ʽ�� ��
��5�����������ĵ缫��ӦΪ ����֤����������Լ�Ϊ ��
��6����д�������ӷ���ʽ ��
(1) MH-e-+OH-=H2O+M (2)Ni(OH)2-e-+OH-=NiOOH+H2O (3)Fe(OH)3 Al(OH)3 ��
��4��NiCl2 + Na2C2O4 + 2H2O = NiC2O4. 2H2O�� + 2NaCl
��5��2Cl��2e-=Cl2�������۵⻯����Һ�������������𰸣���
��6��2Ni(OH)2 + 2OH- + Cl2 = 2Ni(OH)3+ 2Cl-
���������������1���ŵ�ʱ����������������Ӧ�����Ե缫��ӦΪ��MH-e-+OH-=H2O+M��
��2�����ʱ����������������Ӧ�����Ե缫��ӦΪ��Ni(OH)2-e-+OH-=NiOOH+H2O
��3������Ksp���Կ�����pH����ʱ��Fe(OH)3���ȳ�����Ȼ����Al(OH)3�����Ni(OH)2�����Բ�����������ij���II��Fe(OH)3�������ij���III��Al(OH)3��pH1< pH2��
��4�������ܽ�ȿ�֪NiC2O4��2H2O���ܽ����С�����Ԣ۵Ļ�ѧ����ʽΪNiCl2 + Na2C2O4 + 2H2O = NiC2O4. 2H2O�� + 2NaCl��
��5�����ݿ�ͼ��֪DΪNaCl��Һ�����Ե��ʱ������ӦΪ2Cl��2e-=Cl2�����������������õ��۵⻯����Һ��
��6�����ݿ�ͼ��֪CΪNi(OH)2�����Ԣ����ӷ���ʽΪ��2Ni(OH)2+2OH-+Cl2=2Ni(OH)3+
2Cl-��
���㣺��ѧ��Դ��ҵ����
�����������ۺ���ǿ���ѶȽϴ���Ҫ����ѧ���ķ��������ͽ����������Ǹ߿����ȵ�ϰ�⡣

 ���ٴ�����ɽ����ϵ�д�
���ٴ�����ɽ����ϵ�д�����14�֣��±���Ԫ�����ڱ��е�һ���֣�����A��I�����ڱ��е�λ�ã���Ԫ�ط��Ż�ѧʽ�ش��������⣺
 | ��A | | | | | | | 0 |
| 1 | | ��A | ��A | ��A | ��A | ��A | ��A | |
| 2 | | | | D | E | | G | I |
| 3 | A | B | C | | F | | H | |
��2������������ˮ���������ǿ���� ��������ǿ���� �������Ե��� ��
��3��Ҫ֤��A��B��C�Ľ��������ԣ�������ʲôʵ����֤�����Ծ�һ��
ʵ����� ��
ʵ������ ��
�йػ�ѧ����ʽ��
(4)G��H����̬�⻯���ȶ��� > ��˵����Ӧ���ʵķǽ����� > ��
(14��)�����⻯�����MH��Ni�������������ܡ���ȫ������Ⱦ������ЧӦ���۸����ˣ��ѳ�ΪĿǰ��߷�չǰ���ġ���ɫ��Դ�����֮һ������ܷ�ӦΪMH+NiOOH 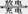 M+Ni(OH)2��MΪ����Ͻ�MHΪ��������ԭ�ӵĴ���Ͻ𡣵������ҺΪŨKOH��Һ��
M+Ni(OH)2��MΪ����Ͻ�MHΪ��������ԭ�ӵĴ���Ͻ𡣵������ҺΪŨKOH��Һ��
(1)д���ŵ�ʱ�ĸ�����Ӧ_________________
(2)���ʱ�������ĵ缫��ӦΪ__________________
����������������Ni(OH)2��̼�ۡ���������Ϳ�����������Ƴɡ�ij��ȤС��Ըõ�ص缫���Ͻ�����Դ�����о������ʵ���������£�
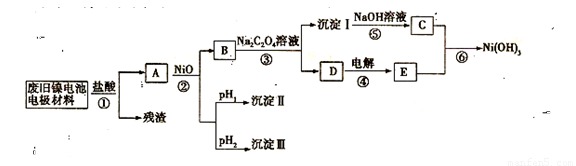
��֪����NiCl2������ˮ��Fe3+��������Ni2+
��ij�¶���һЩ�������������Ksp����������������pH���±���ʾ��
|
M(OH)x |
Ksp |
pH |
|
|
��ʼ���� |
������ȫ |
||
|
Al(OH)3 |
1.9��10-23 |
3.43 |
4.19 |
|
Fe(OH)3 |
3.8��10-38 |
2.53 |
2.94 |
|
Ni(OH)2 |
1.6��10-14 |
7.60 |
9.75 |
�ش��������⣺
��3�������ϱ������жϲ�����������ij�����Ϊ ���������ij���Ϊ��Ϊ__________________���ѧʽ������pH1 pH2�����>������=����<������
��4����֪�ܽ�ȣ�NiC2O4 > NiC2O4��H2O > NiC2O4��2H2O����۵Ļ�ѧ����ʽ�� ��
��5�����������ĵ缫��ӦΪ ����֤����������Լ�Ϊ ��
��6����д�������ӷ���ʽ ��
 ������E������������Ӧˮ���ﷴӦ�Ļ�ѧ����ʽ�� ��
������E������������Ӧˮ���ﷴӦ�Ļ�ѧ����ʽ�� �� �ϵ�أ��˵�صĸ�����Ӧʽ�� ��
�ϵ�أ��˵�صĸ�����Ӧʽ�� ��