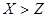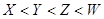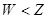��Ŀ����
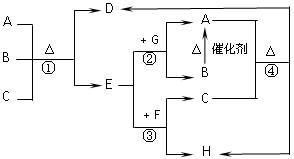
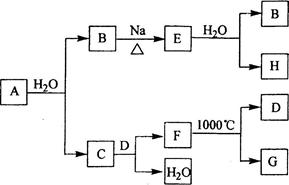
(14��)A��B��C��DΪ���ֵ��ʣ�����ʱ��A��B�����壬C��D�ǹ��塣E��F��G��H��IΪ���ֻ����F����ˮ��EΪ�����Ҽ�����ˮ��Ϊ��ɫ��Һ��G��ˮ�û���ɫ��Һ����������ʼ䷴Ӧ��ת����ϵ��ͼ��ʾ
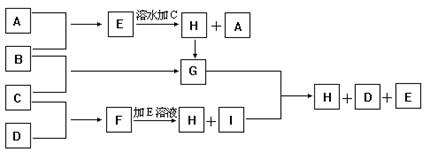
��1��д�����ֵ��ʵĻ�ѧʽ
A____ _____ B__________ C__ ____ D___ _____
��2��д��E��F��H��I�����ӷ���ʽ
��3��д��G+I��H+D+E�Ļ�ѧ����ʽ
��4��ij������B��Ư�ۡ�
��д����Ư�۵Ļ�ѧ����ʽ ��
��Ϊ�ⶨ�ù����Ƶõ�Ư������Ч�ɷֵĺ�����ij��С�����������ʵ�飺��ȡƯ��2.0g����ĥ���ܽ⣬���ó�250mL��Һ��ȡ��25.00mL���뵽��ƿ�У��ټ��������KI��Һ���������ᣨ��ʱ���������ӷ���ʽΪ�� �������á�����ȫ��Ӧ����0.1mol��L-1��Na2S2O3��Һ������Һ�ζ���Ӧ���ɵĵ⣬��֪��ӦʽΪ��2Na2S2O3+I2=Na2S4O6+2NaI������ȥNa2S2O3��Һ20.00mL�����Ư������Ч�ɷֵ���������Ϊ ��������С�������λ����

��1��д�����ֵ��ʵĻ�ѧʽ
A____ _____ B__________ C__ ____ D___ _____
��2��д��E��F��H��I�����ӷ���ʽ
��3��д��G+I��H+D+E�Ļ�ѧ����ʽ
��4��ij������B��Ư�ۡ�
��д����Ư�۵Ļ�ѧ����ʽ ��
��Ϊ�ⶨ�ù����Ƶõ�Ư������Ч�ɷֵĺ�����ij��С�����������ʵ�飺��ȡƯ��2.0g����ĥ���ܽ⣬���ó�250mL��Һ��ȡ��25.00mL���뵽��ƿ�У��ټ��������KI��Һ���������ᣨ��ʱ���������ӷ���ʽΪ�� �������á�����ȫ��Ӧ����0.1mol��L-1��Na2S2O3��Һ������Һ�ζ���Ӧ���ɵĵ⣬��֪��ӦʽΪ��2Na2S2O3+I2=Na2S4O6+2NaI������ȥNa2S2O3��Һ20.00mL�����Ư������Ч�ɷֵ���������Ϊ ��������С�������λ����
��14�֣�ǰ4ÿ��1�֡�����ÿ��2�֣�
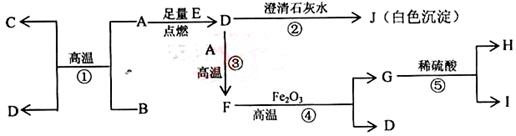
��1��A��H2��B��Cl2��C��Fe��D��S ��2��FeS+2H+=Fe2++H2S�� ��3��2FeCl3+H2S=2FeCl2+S��+2HCl
��4����2Cl2+2Ca(OH)2=CaCl2+Ca(ClO)2+2H2O ��ClO��+2I��+2H+=I2+Cl��+H2O �� 35.75%
��1��A��H2��B��Cl2��C��Fe��D��S ��2��FeS+2H+=Fe2++H2S�� ��3��2FeCl3+H2S=2FeCl2+S��+2HCl
��4����2Cl2+2Ca(OH)2=CaCl2+Ca(ClO)2+2H2O ��ClO��+2I��+2H+=I2+Cl��+H2O �� 35.75%
��

��ϰ��ϵ�д�
�����Ŀ