��Ŀ����
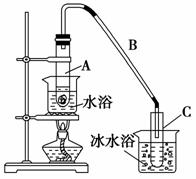
ij��ѧС���������������������װ��(��ͼ)���Ի������Ʊ�����ϩ��
��֪��
| �ܶ�/(g��cm��3) | �۵�/�� | �е�/�� | �ܽ��� | |
| ������ | 0.96 | 25 | 161 | ������ˮ |
| ����ϩ | 0.81 | ��103 | 83 | ������ˮ |
(1)�Ʊ���Ʒ
��12.5 mL�����������Թ�A�У��ټ���1 mLŨ���ᣬҡ�Ⱥ�������Ƭ��������������Ӧ��ȫ�����Թ�C�ڵõ�����ϩ��Ʒ��
��A�����Ƭ��������________������B���˵�������е�������________________________________________________________________��
���Թ�C���ڱ�ˮԡ�е�Ŀ����______________________________________
__________________________________��
(2)�Ʊ���Ʒ
�ٻ���ϩ��Ʒ�к��л������������������ʵȡ����뱥��ʳ��ˮ�������á��ֲ㣬����ϩ��________��(��ϡ����¡�)����Һ����________(������)ϴ�ӡ�
a��KMnO4��Һ��b��ϡH2SO4��c��Na2CO3��Һ
���ٽ�����ϩ����ͼװ��������ȴˮ��________�ڽ��롣����ʱҪ������ʯ�ң�Ŀ����______________________________________________________
________________________________________________________________��
���ռ���Ʒʱ�����Ƶ��¶�Ӧ��________���ң�ʵ���ƵõĻ���ϩ��Ʒ�����������۲��������ܵ�ԭ����________��
a������ʱ��70 �濪ʼ�ռ���Ʒ
b��������ʵ����������
c���Ʊ���Ʒʱ���������Ʒһ������
(3)�������ֻ���ϩ��Ʒ�ʹ�Ʒ�ķ�������������________��
a�������Ը��������Һ��b���ý����ơ�c���ⶨ�е�
��������������ϵ�α��Ʊ�����������ʵ��װ�á�(1)��Ʒ���Ʊ���Ҫ��ֹ��Ӧ�ﱩ�У���Ҫ��ֹ������ӷ���
(2)��Ʒ���Ʊ��ؼ����ڳ��ӣ������漰����Һ��������ϩ�ܶȱ�ˮС�����ϲ㣬ϴ��ʱѡ��KMnO4��Һ����������ϩ�������Ʒ�л��������������ʣ�ϴ��ʱ��������ϡ���ᣬ����Na2CO3��Һ�����������������е�ˮ�ֿ�����ʯ�ҳ�ȥ�����ڻ������ķе�ϵͣ��Ʊ���Ʒʱ���Ʒһ�����������²�����������ֵ��
(3)���־�Ʒ���Ʒ����ѡ��KMnO4����Ϊ���߽Կɱ�KMnO4���������ڴ�Ʒ�к��л������ȣ����������ò������壬�ʿ���Na�������֣��ⶨ�е����ܺܺõ����ֶ��ߡ�
�𰸡�(1)�ٷ�ֹҺ�屩�С��������ڷ�ֹ����ϩ�ӷ���(2)���ϡ�c����g������ˮ�֣�����������������IJ�Ʒ����83 �桡c��(3)b��c

��T��ʱ��ijNaOHϡ��Һ��c(H��)��10��a mol��L��1��c(OH��)��10��b mol��L��1����֪a��b��12�������Һ����μ���pH��c������(T��)����û����Һ�IJ���pH���±���ʾ��
| ��� | NaOH��Һ��� | ������� | ��ҺpH |
| �� | 20.00 | 0.00 | 8 |
| �� | 20.00 | 20.00 | 6 |
������Һ���ǰ�������仯���Բ��ƣ���cΪ(����)
A.1 B��4
C��5 D��6
��ͬ�¶��£��������ȵ����������ܱ������з������淴Ӧ��
N2(g)��3H2(g) 2NH3(g) ��H����92.4 kJ/mol��
2NH3(g) ��H����92.4 kJ/mol��
ʵ������ʼ��ƽ��ʱ���й��������±���
| ������� | ��ʼʱ���������ʵ���/mol | ƽ��ʱ��Ӧ�е������仯 | ||
| N2 | H2 | NH3 | ||
| �� | 1 | 3 | 0 | �ų�����a kJ |
| �� | 2 | 3 | 0 | �ų�����b kJ |
| �� | 2 | 6 | 0 | �ų�����c kJ |
����������ȷ���ǣ� ��
A���ų�������ϵ��a < b < 92.4 B�����������ڷ�Ӧ��ƽ�ⳣ������>��>��
C����ƽ��ʱ�����������������>�� D��N2��ת���ʣ���>��>��
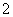 ��SO
��SO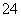 B��H2SO4===2H����S
B��H2SO4===2H����S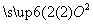 4
4