��Ŀ����
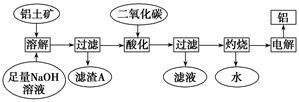
����һ��Ӧ�ù㷺�Ľ�������ҵ����Al2O3�ͱ���ʯ��Na3AlF6��������ڵ���Ƶá����������Ҫ�ɷ���Al2O3��SiO2������������NaOH��Һ�����ʡ���������������Al2O3���������£�
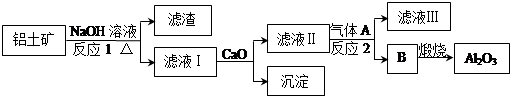
�ش��������⣺
��1��д����Ӧ1�Ļ�ѧ����ʽ ��
��2����Һ���м���CaO���ɵij����� ����Ӧ2�����ӷ���ʽΪ ��
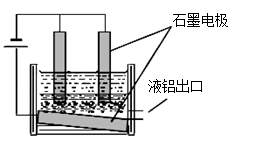
��3����������Ļ�ѧ����ʽ�� ����ʯīΪ�缫�����������Ļ������ijɷ��� ��
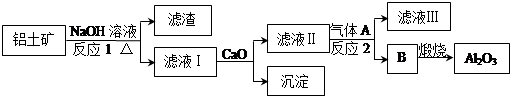
�ش��������⣺
��1��д����Ӧ1�Ļ�ѧ����ʽ ��
��2����Һ���м���CaO���ɵij����� ����Ӧ2�����ӷ���ʽΪ ��
��3����������Ļ�ѧ����ʽ�� ����ʯīΪ�缫�����������Ļ������ijɷ��� ��
��1��2NaOH��SiO2=Na2SiO3��H2O��2�֣� 2NaOH��Al2O3=2NaAlO2��H2O��2�֣�
��2��CaSiO3��1�֣�
2AlO2��+CO2+3H2O=2Al(OH)3��+CO32�� ��AlO2��+CO2+2H2O=Al(OH)3��+HCO3����2�֣�
��3��2Al2O3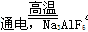 4Al��3O2����2�֣� O2��CO2��CO����2�֣�
4Al��3O2����2�֣� O2��CO2��CO����2�֣�
��2��CaSiO3��1�֣�
2AlO2��+CO2+3H2O=2Al(OH)3��+CO32�� ��AlO2��+CO2+2H2O=Al(OH)3��+HCO3����2�֣�
��3��2Al2O3
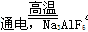 4Al��3O2����2�֣� O2��CO2��CO����2�֣�
4Al��3O2����2�֣� O2��CO2��CO����2�֣������������1��������������������ﶼ������ǿ����Һ�������κ�ˮ����2���������Ǽ����������ˮ��Ӧ�����������ƣ����������������ӽ�ϳɹ���Ƴ�����ƫ��������Һͨ�����������CO2��������ȡ����������������3�������������������ȡ����������ӦʽΪ2O2����2e��=O2����������C��O2��Ӧ����CO2��CO��

��ϰ��ϵ�д�
 ��ʦ����ָ���ο�ʱϵ�д�
��ʦ����ָ���ο�ʱϵ�д�
�����Ŀ