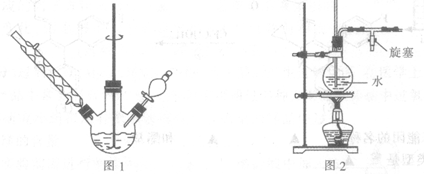
��Ŀ����
ij������Ni���ϴ�������Ҫ����Ni��������Al��Al203��Fe�������������ᡢ������ʡ�����������������������ʽ��ȫ����ʱ��Һ��pH���£�
���Ӻ����ϴ������Ƶ�NiSO4��7H2O���壬���������£�
�����������Ϣ������ͼ���ش��������⣺
��1�����������Ŀ���dz�ȥ�����ϴ����е�___ _��
��2���������ʱ�����������___ _���������Һ���п��ܺ��еĽ���������___ ���������ӷ��ű�ʾ����
��3������pHΪ2��3��Ŀ����___ _��
��4������A�IJ���˳���Ǽ���Ũ������ȴ��____��____��
��5��NiSO4��ǿ����Һ����NaC1O���������Ƶü������ӵ�ص缫����NiOOH���÷�Ӧ�����ӷ���ʽΪ________��
��13�֣���1��Al��Al2O3��2�֣� ��2��H2SO4��2�֣���Ni2����Fe2����2�֣�
��3����ֹ��Ũ���ᾧ������Ni2+ˮ�⣨2�֣� ��4���ᾧ�����ˣ�2�֣�
��5��2Ni2+��ClO-��4OH-��2NiOOH����Cl-��H2O��3�֣�
���������������1�����������������Ϊ�˳�ȥ������������Al2O3�����������������Ժ�ǿ�Ӧ���ܽ�õ�ƫ�����Σ���Ӧ�����ӷ���ʽ�ֱ�Ϊ2Al+2OH-+2H2O��2AlO2-+3H2����Al2O3+2OH-��2AlO2-+3H2O��
��2���������ʱ��Ҫ���ܽ��������������ʼ�������������Ʊ�Ŀ���ǵõ�NiSO4?7H2O����˼������������µ����ʣ�������Ҫ������������������������ϡ���ᷴӦ����������������������Һ�������Һ���п��ܺ��еĽ���������Ni2+��Fe2+��
��3����������Һ��Ҫ����Ũ���ᾧ������Ϊ��ֹ������ˮ����������������������Ҫ������ҺpH��
��4������A�IJ���˳���Ǽ���Ũ������ȴ���ᾧ�����ˡ�
��5��NiSO4��ǿ����������NaClO���������Ƶü������ӵ�ص缫����NiOOH����˸÷�Ӧ�����ӷ���ʽ��2Ni2+��ClO-��4OH-��2NiOOH����Cl-��H2O��
���㣺�������ʵķ�����ᴿ����ѧʵ�����������ʵ�鷽������Լ�ʵ���������Ƶ��ۺ�Ӧ�õ�

S2Cl2�ǹ�ҵ�ϳ��õ�����ʵ�����Ʊ�S2Cl2�ķ�����2�֣�
�� CS2+3Cl2 CCl4+S2Cl2���� 2S+Cl2
CCl4+S2Cl2���� 2S+Cl2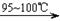 S2Cl2��
S2Cl2��
��֪S2Cl2����Ԫ����+1�ۣ�����ʽ��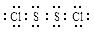 �������ȶ�����ˮ������᪻���Ӧ��һ������Ԫ�ؼ�̬���ߣ�һ���ֽ��ͣ�����Ӧ�漰�ļ������ʵ��۷е����£�
�������ȶ�����ˮ������᪻���Ӧ��һ������Ԫ�ؼ�̬���ߣ�һ���ֽ��ͣ�����Ӧ�漰�ļ������ʵ��۷е����£�
| ���� | S | CS2 | CCl4 | S2Cl2 |
| �е�/�� | 445 | 47 | 77 | 137 |
| �۵�/�� | 113 | -109 | -23 | -77 |
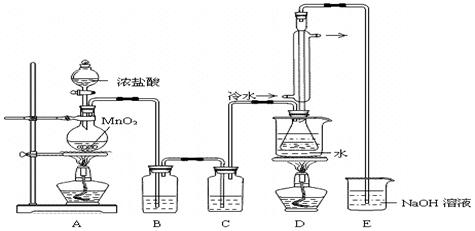
ʵ������������װ���Ʊ�S2Cl2�����ּг���������ȥ����
�ش��������⣺
��1��װ��B��C�в������������ƣ� ����Ӧԭ������д������ţ��� ��
��2��ʵ���������Լ�ͨ������36.5%��Ũ��Һ������ϡ����������� ��
��3��D������������������˫�����ã�����ȴˮ��������������������������ͬ����ͼ����������ȴ��ʽ��Ӧ�������и��л�ѧ�� ʵ�顣
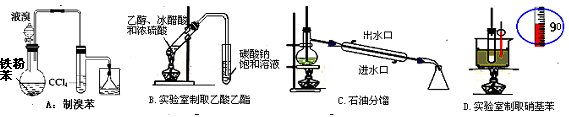
A��ʯ�ͷ��� B����ȡ�屽 C����ȡ�������� D���Ʊ���˾ƥ��
��4��Bװ����ʢ�ŵ��� ����Ӧ���������ƿ�ڻ�����з������Ʒ�ķ����� ��D�в�����ˮԡ���ȵ�ԭ���� ��
��5��A��������װ��ʱ���ź�����̨��Ӧ�ȹ̶� �����������ƣ�������װ��װ����Ϻ�Ӧ�Ƚ��� �������Լ���ʵ����ϣ�A�в��ٲ�������ʱ���ɲ��װ�á����ʱ�����ȵIJ���Ӧ���� ��
��6��ʵ������У���ȱ��Cװ�ã����ֲ�Ʒ���Dz��壬���ָ������ԭ����û�ѧ����ʽ��ʾΪ ��ʵ����ϣ�����ʣ��Ũ���ᵹ��E�ձ�����������β��������������Һ���ʱ����������������ɫ�̼�����������������������ԭ���ǣ� �������ӷ���ʽ��ʾ����
Ư����һ�ֳ��õ���������
��1����ҵ������Ư�۷�Ӧ�Ļ�ѧ����ʽΪ��________________ __��Ư�۵���Ч�ɷ�Ϊ ��
��2��ij̽��С����г��Ϲ�����һ����װ�����Ư�ۣ��Ը�Ư�۵ijɷֽ���̽�������������Լ������ʵ�鷽��������ʵ�顣���ڴ�������ʵ�鱨�档
��ѡ�Լ���2mol��L��1NaOH��Һ��2mol��L��1HCl��Һ��2mol��L��1HNO3��Һ��0.5mol��L��1BaCl2��Һ��0.01mol��L��1AgNO3��Һ������ʯ��ˮ��ʯ����Һ����̪��Һ������ˮ��
| ʵ�鲽�� | Ԥ����������� |
| ����1��ȡ����Ư��������������ˮ����ֽ��裬���ã����ˣ��ó�������Һ�� | |
| ����2���������������2mol��L��1HCl��Һ��������������ͨ�� | ���� ���ۣ� |
| ����3��ȡ��Һ��װA��B��֧�Թܡ���A�Թܣ� | ������Һ�ȱ��ɫ��Ȼ����ɫ�� ���ۣ� |
| ����4����B�Թܣ� | ��������ɫ������ ���ۣ� |
��3��̽��С��Ϊ�ⶨƯ����Ca(ClO)2�ĺ�������ȡƯ��bg��ˮ�ܽ�����Ƴ�100mL��Һ��ȷ��ȡ25.00mL����ƿ���������������KI��Һ����ַ�Ӧ����Һ�е��������0.1000mol/L��Na2S2O3��Һ�ζ����ζ�2�Σ�ƽ������Na2S2O3��Һ20.00mL�����Ư����Ca(ClO)2����������Ϊ_____________ _����ֻ����ʽ���������㣬��֪��Mr[Ca(ClO)2]="143" ��Ca(ClO)2+4HCl=2Cl2��+CaCl2+2H2O��2Na2S2O3+I2=Na2S4O6+2NaI��
NaCl��NaClO�����������¿ɷ�����Ӧ��ClO��+Cl��+2H+ = Cl2��+H2O��ijѧϰС�����о�����Һ(��Ҫ�ɷ�ΪNaCl��NaClO)�ı��������
��1��������Һ��NaClO�����տ����е�CO2����NaHCO3��HClO�����ʡ�д����ѧ��Ӧ����ʽ ��
��2��ȡ��������Һ�����Թ��У���������һ��Ũ�ȵ����ᣬ������ų���ͨ������װ�ü�������ijɷֿ����ж�����Һ�Ƿ���ʡ�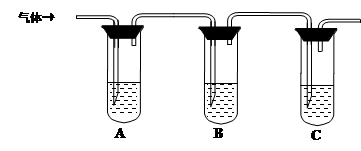
��ѡ�Լ���98%Ũ���ᡢ1%Ʒ����Һ��1.0 mol��L��1 KI-������Һ��1.0 mol��L��1NaOH������ʯ��ˮ������NaCl��Һ
���������ʵ�鷽����
| �����Լ� | Ԥ������ͽ��� |
| �Թ�A�м������� �� �Թ�B�м�1%Ʒ����Һ�� �Թ�C�мӢ� �� | ��A����Һ����ɫ��B����Һ����ɫ��C����Һ����ǡ�������Һ���ֱ��ʣ� �� ������Һδ���ʣ� �� ������Һ��ȫ���ʡ� |
��3���õζ����ⶨ����Һ��NaClO��Ũ�ȡ�ʵ�鲽�����£�
����ȡ 25.00mL����Һ������ƿ�У����������a mol��L��1 Na2SO3��Һb mL��
�ڵζ���������c mol��L��1������KMnO4��Һװ�� ������ʽ���ʽ���ζ����У�KMnO4��ʣ���Na2SO3������Ӧ������Һ����ɫ���dz��ɫ���ұ��ְ�����ں�ɫ����ʱ��ֹͣ�ζ�����¼���ݡ��ظ��ζ�ʵ��2�Σ�ƽ����������KMnO4��Һv mL��
�ζ��������漰�ķ�Ӧ�У�NaClO + Na2SO3 = NaCl+ Na2SO4 ��
2KMnO4 + 5Na2SO3+ 3H2SO4 = K2SO4 + 2MnSO4 + 5Na2SO4 + 3H2O
�ۼ��㡣����Һ��NaClO��Ũ��Ϊ mol��L��1���ú�a��b��c��v�Ĵ���ʽ��ʾ����
ijͭ��ʯ��ͭԪ�غ����ϵͣ��Һ�������þ���Ƶ��������ӡ�ijС����ʵ�������ý���-��ȡ���Ʊ�����ͭ��
��1������IΪ_______������II�õ��IJ����������ձ�_______
��2������II������III����ҪĿ����_______������ͭԪ�ء�
��3��С���Ա����CuSO4��Һ��Na2CO3��Һ��Ϸ�Ӧ���Ʊ�������ľ�ķ�����Cu2(OH)2CO3����Һ�����ʵ�鷢��������ɫ����Һ��ɫ���в��죬�������ϱ��������������������Ʋ�ͬʹ���л��н϶�Cu(OH)2��Cu4(OH)6SO4��
��֪Cu(OH)2��Cu2(OH)2CO3��Cu4((OH)6SO4��������ˮ����������ֽ��¶�����Ϊ 80�桢200�桢300�档
���ʵ���������Һ�ɷ֣���ɱ������ݡ�
��ѡ�Լ���2mol?L��1HCl��1 mol?L��1H2SO4��0.1 mol?L��1NaOH��0.1 mol?L��1 BaCl2������ˮ����������Ʒ��ѡ��
| ʵ�鲽�� | Ԥ������ͽ��� |
| ����1��ȡ��������Һ�����ˣ����ϴ�Ӻ�ȡ�������Թ��У�_______________________________________________________________ | ˵������Һ�л�__________________________________________,��Cu4( OH)6SO4�� |
| ����2����ȡ��������Һ���Թ��У�____________________________ | ______________�� ˵������Һ�л���Cu( OH) 2�� |
��4������ʵ����Ҫ100mL 0.5 mol?L��1��CuSO4��Һ������ʱ���ȡ_______gCuSO4?5H2O (��ѧʽ����250)��
ʵ������ֻ���ռ���ᡢͭƬ������ʯ������ˮ�����Լ�����Ƿȱ�Լ��ĽǶ�����(ʵ��������ȫ)�������е�ʵ����Ŀ��( )
| A����ȡ���� | B����ȡ̼���� | C����ȡ�Ȼ�ͭ | D���ⶨ�����Ũ�� |