��Ŀ����
�����������գ�
��1����SO2��SO3�з��Ӹ�����Ϊ1��1 ʱ��ԭ������֮��Ϊ
��2����5mol/L��Mg��NO3��2��Һa mLϡ����b mL��ϡ�ͺ���Һ��NO3-�����ʵ���Ũ����
mol/L
mol/L��
��3���кͺ�0.2molHCl��ϡ���ᣬ��NaOH�����ʵ���Ϊ
��1����SO2��SO3�з��Ӹ�����Ϊ1��1 ʱ��ԭ������֮��Ϊ
3��4
3��4
������֮��Ϊ4��5
4��5
����2����5mol/L��Mg��NO3��2��Һa mLϡ����b mL��ϡ�ͺ���Һ��NO3-�����ʵ���Ũ����
| 10a |
| b |
| 10a |
| b |
��3���кͺ�0.2molHCl��ϡ���ᣬ��NaOH�����ʵ���Ϊ
0.2mol
0.2mol
�������KOH��������KOH������Ϊ11.2g
11.2g
����������1������n=
=
��Ϸ�����ɼ��㣻
��2����Һϡ��ǰ�����ʵ����ʵ������䣻
��3�����ݷ�ӦH++OH-=H2O���㣮
| N |
| NA |
| m |
| M |
��2����Һϡ��ǰ�����ʵ����ʵ������䣻
��3�����ݷ�ӦH++OH-=H2O���㣮
����⣺��1��1��SO2�����к���3��ԭ�ӣ�1��SO3�����к���4��ԭ�ӣ���SO2��SO3�з��Ӹ�����Ϊ1��1 ʱ��ԭ������֮��Ϊ3��4������n=
=
��֪����������ͬʱ�����ʵ�����ͬ����������Ħ��������֮�ȣ�������֮��Ϊ��64��80=4��5���ʴ�Ϊ��3��4��4��5��
��2��5mol/L��Mg��NO3��2��Һ�У�c��NO3-��=2c��Mg��NO3��2��=10mol/L��
��5mol/L��Mg��NO3��2��Һa mLϡ����b mL��
����10mol/L��a��10-3L=c��b��10-3L��
ϡ�ͺ���Һ��NO3-�����ʵ���Ũ����c=
mol/L��
�ʴ�Ϊ��
mol/L��
��3���ɷ�ӦH++OH-=H2O��֪��
n��NaOH��=n��HCl��=0.2mol��
n��KOH��=n��HCl��=0.2mol��
m��KOH��=0.2mol��56g/mol=11.2g��
�ʴ�Ϊ��0.2mol��11.2g��
| N |
| NA |
| m |
| M |
��2��5mol/L��Mg��NO3��2��Һ�У�c��NO3-��=2c��Mg��NO3��2��=10mol/L��
��5mol/L��Mg��NO3��2��Һa mLϡ����b mL��
����10mol/L��a��10-3L=c��b��10-3L��
ϡ�ͺ���Һ��NO3-�����ʵ���Ũ����c=
| 10a |
| b |
�ʴ�Ϊ��
| 10a |
| b |
��3���ɷ�ӦH++OH-=H2O��֪��
n��NaOH��=n��HCl��=0.2mol��
n��KOH��=n��HCl��=0.2mol��
m��KOH��=0.2mol��56g/mol=11.2g��
�ʴ�Ϊ��0.2mol��11.2g��
���������⿼�����ʵ����ļ��㣬��Ŀ�ѶȲ���ע����ؼ��㹫ʽ�����ã�

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д� Ŀ�����ϵ�д�
Ŀ�����ϵ�д�
�����Ŀ
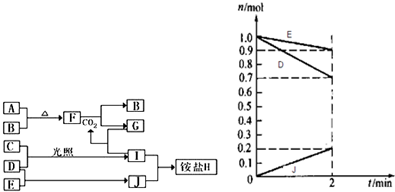
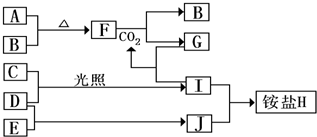
 2NH3
2NH3