��Ŀ����
��ͼ�Dz��ֶ�����Ԫ�صĵ��ʼ��仯���������Һ����ת����ϵ����֪B��C��D��E�Ƿǽ������ʣ����ڳ��³�ѹ�¶������壻������G����ɫ��ӦΪ��ɫ��������I��Jͨ��״���³���̬��
�����������գ�
��1��H�Ļ�ѧʽ
��2��A��B �ڼ��������·�Ӧ����Ҫ������
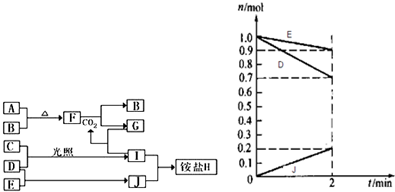
��3��ij�¶�ʱ����2L�����У�D��E��J�������ʵ����ʵ�����ʱ��仯��������ͼ��ʾ����ͼ�����ݷ������÷�Ӧ�Ļ�ѧ����ʽΪ

�����������գ�
��1��H�Ļ�ѧʽ
NH4Cl
NH4Cl
���侧����������ѧ������Ϊ���Ӽ������ۼ�
���Ӽ������ۼ�
������H �������ӵķ�����ȡ����H��Ʒ�����Թܣ���������������Һ�����ȣ�������ʹʪ��ĺ�ɫʯ����ֽ���������壬֤����笠�����
ȡ����H��Ʒ�����Թܣ���������������Һ�����ȣ�������ʹʪ��ĺ�ɫʯ����ֽ���������壬֤����笠�����
����2��A��B �ڼ��������·�Ӧ����Ҫ������
���ҷ�Ӧ������Ϊ��ɫ�����ɵ���ɫ�Ĺ���
���ҷ�Ӧ������Ϊ��ɫ�����ɵ���ɫ�Ĺ���
��F��H2O��Ӧ�Ļ�ѧ����ʽΪ2Na2O2+2H2O=4NaOH+O2��
2Na2O2+2H2O=4NaOH+O2��
��д��G��Һ�м��������I��Һ��Ӧ�����ӷ���ʽCO32-+2H+=CO2��+H2O
CO32-+2H+=CO2��+H2O
����3��ij�¶�ʱ����2L�����У�D��E��J�������ʵ����ʵ�����ʱ��仯��������ͼ��ʾ����ͼ�����ݷ������÷�Ӧ�Ļ�ѧ����ʽΪ
N2+3H2 2NH3
2NH3
 2NH3
2NH3N2+3H2 2NH3
2NH3
����Ӧ��ʼ��2min����J��ʾ��ƽ����Ӧ����Ϊ 2NH3
2NH30.05mol/L?min
0.05mol/L?min
������������ת����ϵ�еķ�Ӧ����������C+D=I�ķ�Ӧ�����ǹ��գ��ƶ�Ϊ�����������ķ�Ӧ��I+J=���H��˵��I��HCl��J��NH3��DΪH2��C��Cl2��EΪN2��������G����ɫ��ӦΪ��ɫ˵������Ԫ�أ�G+I=CO2��֤��GΪ̼���Σ�B�Ƿǽ���������F�Ͷ�����̼��Ӧ���ɵģ��ƶ�FΪNa2O2��BΪO2��AΪNa�������ƶϳ������ʽ��з����ش����⣮
����⣺��֪B��C��D��E�Ƿǽ������ʣ����ڳ��³�ѹ�¶������壻������G����ɫ��ӦΪ��ɫ��������I��Jͨ��״���³���̬������ת����ϵ�еķ�Ӧ����������C+D=I�ķ�Ӧ�����ǹ��գ��ƶ�Ϊ�����������ķ�Ӧ��I+J=���H��˵��I��HCl��J��NH3��DΪH2��C��Cl2��EΪN2��������G����ɫ��ӦΪ��ɫ˵������Ԫ�أ�G+I=CO2��֤��GΪ̼���Σ�B�Ƿǽ���������F�Ͷ�����̼��Ӧ���ɵģ��ƶ�FΪNa2O2��BΪO2��AΪNa��
��1��H�Ļ�ѧʽΪNH4Cl��������������ѧ������Ϊ���Ӽ������ۼ�������H��NH4Cl���������ӣ�NH4+���ķ���Ϊ��ȡ����H ��Ʒ�����Թܣ���������������Һ�����ȣ�������ʹʪ��ĺ�ɫʯ����ֽ���������壬֤����笠����ӣ�
�ʴ�Ϊ��NH4Cl�����Ӽ������ۼ���ȡ����H ��Ʒ�����Թܣ���������������Һ�����ȣ�������ʹʪ��ĺ�ɫʯ����ֽ���������壬֤����笠����ӣ�
��2��A��Na����B��O2�� �ڼ��������·�Ӧ����Ҫ�����ǣ����ҷ�Ӧ������Ϊ��ɫ�����ɵ���ɫ�Ĺ��壻F��Na2O2����H2O��Ӧ�Ļ�ѧ����ʽΪ��2Na2O2+2H2O=4NaOH+O2����G��NH4Cl����Һ�м��������I��HCl����Һ��Ӧ�����ӷ���ʽ��CO32-+2H+=CO2��+H2O��
�ʴ�Ϊ�����ҷ�Ӧ������Ϊ��ɫ�����ɵ���ɫ�Ĺ��壻2Na2O2+2H2O=4NaOH+O2����CO32-+2H+=CO2��+H2O��
��3����ͼ�����ݷ��������ķ�ӦΪD+E=J��D��E�������ʵ���Ϊn��H2��=1.0mol-0.7mol=0.3mol��n��N2��=1.0mol-0.9mol=0.1mol������J�����ʵ���Ϊ��n��NH3��=0.2mol����Ӧ���ʵ���֮��Ϊn��H2����n��N2����n��NH3��=0.3mol��0.1mol��0.2mol=3��1��2���÷�Ӧ�Ļ�ѧ����ʽΪN2+3H2 2NH3����J��NH3����ʾ�ķ�Ӧ����=
2NH3����J��NH3����ʾ�ķ�Ӧ����=
=0.05mol/L?min��
�ʴ�Ϊ��N2+3H2 2NH3��0.05mol/L?min��
2NH3��0.05mol/L?min��
��1��H�Ļ�ѧʽΪNH4Cl��������������ѧ������Ϊ���Ӽ������ۼ�������H��NH4Cl���������ӣ�NH4+���ķ���Ϊ��ȡ����H ��Ʒ�����Թܣ���������������Һ�����ȣ�������ʹʪ��ĺ�ɫʯ����ֽ���������壬֤����笠����ӣ�
�ʴ�Ϊ��NH4Cl�����Ӽ������ۼ���ȡ����H ��Ʒ�����Թܣ���������������Һ�����ȣ�������ʹʪ��ĺ�ɫʯ����ֽ���������壬֤����笠����ӣ�
��2��A��Na����B��O2�� �ڼ��������·�Ӧ����Ҫ�����ǣ����ҷ�Ӧ������Ϊ��ɫ�����ɵ���ɫ�Ĺ��壻F��Na2O2����H2O��Ӧ�Ļ�ѧ����ʽΪ��2Na2O2+2H2O=4NaOH+O2����G��NH4Cl����Һ�м��������I��HCl����Һ��Ӧ�����ӷ���ʽ��CO32-+2H+=CO2��+H2O��
�ʴ�Ϊ�����ҷ�Ӧ������Ϊ��ɫ�����ɵ���ɫ�Ĺ��壻2Na2O2+2H2O=4NaOH+O2����CO32-+2H+=CO2��+H2O��
��3����ͼ�����ݷ��������ķ�ӦΪD+E=J��D��E�������ʵ���Ϊn��H2��=1.0mol-0.7mol=0.3mol��n��N2��=1.0mol-0.9mol=0.1mol������J�����ʵ���Ϊ��n��NH3��=0.2mol����Ӧ���ʵ���֮��Ϊn��H2����n��N2����n��NH3��=0.3mol��0.1mol��0.2mol=3��1��2���÷�Ӧ�Ļ�ѧ����ʽΪN2+3H2
 2NH3����J��NH3����ʾ�ķ�Ӧ����=
2NH3����J��NH3����ʾ�ķ�Ӧ����=
| ||
| 2min |
�ʴ�Ϊ��N2+3H2
 2NH3��0.05mol/L?min��
2NH3��0.05mol/L?min�����������⿼��������ת����ϵ���������ʵ�Ӧ�ã���Ҫ���鹤ҵ�ϳɰ�����ҵ�Ʊ����ᣬ�Ƽ��仯�������ʵ�Ӧ�ã���ѧ��Ӧ���ʼ��㣬��ѧ����ʽ�����жϣ�

��ϰ��ϵ�д�
 �߽�������ϵ�д�
�߽�������ϵ�д�
�����Ŀ
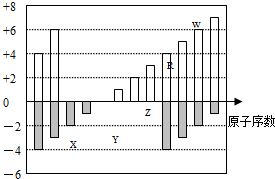 ��2012?�㶫����ͼ�Dz��ֶ�����Ԫ�ػ��ϼ���ԭ�������Ĺ�ϵͼ������˵����ȷ���ǣ�������
��2012?�㶫����ͼ�Dz��ֶ�����Ԫ�ػ��ϼ���ԭ�������Ĺ�ϵͼ������˵����ȷ���ǣ�������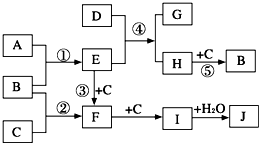 ��2009?����һģ����ͼ�Dz��ֶ�����Ԫ�صĵ��ʼ��仯�����ת����ϵͼ���йط�Ӧ�����������ɵ�H2O����ȥ����
��2009?����һģ����ͼ�Dz��ֶ�����Ԫ�صĵ��ʼ��仯�����ת����ϵͼ���йط�Ӧ�����������ɵ�H2O����ȥ����
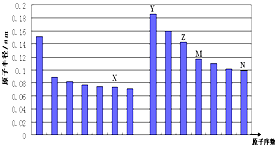 ��ͼ�Dz��ֶ�����Ԫ��ԭ�Ӱ뾶��ԭ�������Ĺ�ϵͼ������˵������ȷ���ǣ�������
��ͼ�Dz��ֶ�����Ԫ��ԭ�Ӱ뾶��ԭ�������Ĺ�ϵͼ������˵������ȷ���ǣ������� ��ͼ�Dz��ֶ�����Ԫ�صĵ����۵�ı仯ͼ�����ݴ�ͼ����д���пո�
��ͼ�Dz��ֶ�����Ԫ�صĵ����۵�ı仯ͼ�����ݴ�ͼ����д���пո�