��Ŀ����
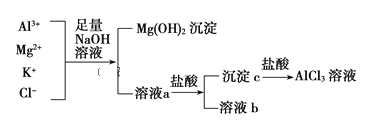
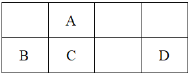
����Ŀ��������Ԫ��A��B��C��D��λ����ͼ��ʾ��������D����Ϊ����ɫ���塣
�ش��������⣺
��1��CԪ�������ڱ��е�λ��___��������Ȼ���г�������������___���塣
��2��A�ĵ����ڳ����������ȼ�յõ��IJ���Ľṹʽ��___���������__���ӣ��������������Ǽ���������
��3������Ԫ���п����ں��պ���Ͻ���ϵ��Ʊ���������������ˮ����ĵ��뷽��ʽΪ___��
��4����Bͬ��������õĽ�����___�����֤������B���ã�������йصķ���ʽ����˵����__��
���𰸡��������ڵڢ�A�� ԭ�� O��C��O �Ǽ��� H++AlO2-+H2O![]() Al(OH)3
Al(OH)3![]() Al3++3OH- Na NaOH�ļ���ǿ��Al(OH)3��Al(OH)3+NaOH=NaAlO2+2H2O
Al3++3OH- Na NaOH�ļ���ǿ��Al(OH)3��Al(OH)3+NaOH=NaAlO2+2H2O
��������
������D����Ϊ����ɫ���壬˵��DΪ���ʣ�����ͼ���ɵó�AΪC��BΪAl��CΪSi��
��CԪ��Ϊ��Ԫ�أ�14��Ԫ�أ������ڱ��е�λ�õ������ڵ���A�壬�ʴ�Ϊ�������ڵ���A�壻
������Ȼ���г�����������Ϊ�������裬��Ϊԭ�Ӿ��壬�ʴ�Ϊԭ�Ӿ��壻
��AΪCԪ�أ������ڳ����������ȼ�յõ��IJ���Ϊ������̼����ṹʽ��O��C��O���ʴ�ΪO��C��O��
������̼��ֱ���η��ӣ������������������غϣ�������ǷǼ��Է��ӣ��ʴ�Ϊ�Ǽ��ԣ�
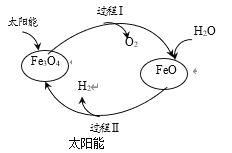
������Ԫ���п����ں��պ���Ͻ���ϵ��Ʊ�����Ӧ��ΪAlԪ�أ�������������ˮ����Ϊ�������������������ĵ�������ʽ����ͼ�ʽ���룬����뷽��ʽΪH++AlO2��+H2O![]() Al(OH)3
Al(OH)3![]() Al3++3OH�����ʴ�ΪH++AlO2��+H2O
Al3++3OH�����ʴ�ΪH++AlO2��+H2O![]() Al(OH)3
Al(OH)3![]() Al3++3OH����
Al3++3OH����
����Bͬ��������õĽ���������ߵĽ���Na��֤��Na��Al���ã���֤��NaOH�ļ���ǿ��Al(OH)3�������������������ԣ�����ǿ���������֤������Al(OH)3+NaOH=NaAlO2+2H2O���ʴ�ΪNaOH�ļ���ǿ��Al(OH)3��
Al(OH)3+NaOH=NaAlO2+2H2O��

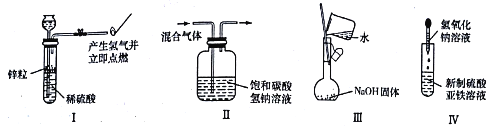
����Ŀ��Ϊ̽��Na��C02��Ӧ���ij��ѧ��ȤС�鰴��ͼװ�ý���ʵ�顣
��֪��CO+2Ag��NH3��2OH=2Ag��+ ��NH4��2CO3+2NH3

�ش��������⣺
(1)д��A�з�Ӧ�����ӷ���ʽ_____________________________________��
(2)����X��������____________________��B�е���ҺΪ_____________________��
(3)�ȳ���Ӳ�ʲ����ܵ�����Ϊmlg������Ʒװ��Ӳ�ʲ������У��Ƶ���Ʒ��Ӳ�ʲ����ܵ���������m2g.�ٽ�������ʵ�����������ȷ˳����____________�����ţ���
a.��ȼ�ƾ��ƣ����� b.Ϩ��ƾ��� c.�ر�K1��K2
d.��K1��K2��ͨ��C02��E�г��ֻ��� e.����Ӳ�ʲ����� f.��ȴ������
�ظ������������裬ֱ��Ӳ�ʲ����ܺ��أ��Ƶ�����Ϊm3g��
(4)����Ӳ�ʲ�����һ��ʱ�䣬�۲쵽��������
���ƿ�����ڣ����ڳɽ���С��
���������ȣ���Ѹ��ȼ�գ�������ɫ���档��Ӧ��ȫ�����д�����ɫ���ʣ�
��F���Թ��ڱ����������ʲ�����
���������������ԭ����______________________________________��
(5)̽�����������Ԫ��Na�Ĵ�����ʽ
����һ��ֻ��Na2CO3��
�������ֻ��Na2O��
��������Na2O��Na2CO3����
�������ʵ����ƣ���֤��������:
���� | ���� | ���� |
1 | ��Ӳ�ʲ������еĹ����������ˮ����ˣ� | ����һ���� |
2 | ������1������Һ��_________________________________�� ����____________________________________�� |
m1 | m2 | m3 |
66.7g | 69.0g | 72.lg |
(6)��������ʵ�������±�ʵ�����ݣ�д��Na��CO2��Ӧ���ܻ�ѧ����ʽ____________________________________________��
����Ŀ���������ʵ�����X��Y�������ij�ܱ������У���һ�������£��������·�Ӧ���ﵽƽ�⣺
X(g)+ 3Y(g) ![]() 2Z(g) ��H<0 ���ı�ij��������ά��������ֱ���µ�ƽ��ʱ���±��й�����ƽ����ԭƽ��ıȽ���ȷ����
2Z(g) ��H<0 ���ı�ij��������ά��������ֱ���µ�ƽ��ʱ���±��й�����ƽ����ԭƽ��ıȽ���ȷ����
ѡ�� | �ı����� | ��ƽ����ԭƽ��Ƚ� |
A | �����¶� | X��ת���ʱ�С |
B | ����ѹǿ | X��Ũ�ȱ�С |
C | ����һ����Y | Y��ת�������� |
D | ʹ���ʵ����� | X�����������С |
A. A B. B C. C D. D