��Ŀ����
��һ����ɫ���壬���ܺ���CaCO3��Na2SO4��KNO3��CuSO4��BaCl2���������е�һ�ֻ��֡��ֽ�������ʵ�飺
��1��ȡ���������ĩ�ӵ�����ˮ�У��õ���ɫ�������ϲ�Ϊ��ɫ��Һ��
��2�����������м�������ϡ���ᣬ��ɫ������ȫ��ʧ���������ݲ�����
��3��ȡ�������е���Һ�μ�Ba(NO3)2��Һ���а�ɫ�������ɣ��ټ���ϡ���ᣬ�������ܡ�
��������ʵ�������жϣ��ð�ɫ������һ������__________________��һ��������________�����ܺ���_____________��
�����֤���ܴ��ڵ�����
��1��ȡ���������ĩ�ӵ�����ˮ�У��õ���ɫ�������ϲ�Ϊ��ɫ��Һ��
��2�����������м�������ϡ���ᣬ��ɫ������ȫ��ʧ���������ݲ�����
��3��ȡ�������е���Һ�μ�Ba(NO3)2��Һ���а�ɫ�������ɣ��ټ���ϡ���ᣬ�������ܡ�
��������ʵ�������жϣ��ð�ɫ������һ������__________________��һ��������________�����ܺ���_____________��
�����֤���ܴ��ڵ�����
һ������CaCO3��Na2SO4����2�֣���һ��������CuSO4��BaCl2����2�֣������ܺ���KNO3����1�֣���
ȡ����������ɫ��Ӧ������ɫ�ܲ������������ɫ��֤����KNO3��2�֣�
ȡ����������ɫ��Ӧ������ɫ�ܲ������������ɫ��֤����KNO3��2�֣�
������������ݣ�1�����õ���ɫ�������ϲ�Ϊ��ɫ��Һ����˵����Һ�в�����CuSO4�����г�������ΪBaSO4��CaCO3�����ݣ�2���������ɫ������ʧ��ȷ����������BaSO4ӦΪCaCO3������(3)�ж���Һ�к���SO42-����ԭ�����к���Na2SO4������ȷ������KNO3��������ɫ��Ӧ��������ӣ�ȷ��KNO3�Ƿ���ڡ�

��ϰ��ϵ�д�
�����Ŀ
 ����
����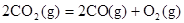 ��Ӧ��
��Ӧ��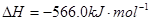
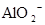 ����Һ�У�K����
����Һ�У�K����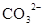 ��
��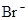 ��
��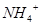 �ɹ���
�ɹ���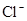 ��
��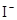 ��
��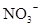 ��
��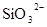 �������֣����������������������ݣ���Һ��ɫ�����������������3�֣���ԭ��Һ��һ����
�������֣����������������������ݣ���Һ��ɫ�����������������3�֣���ԭ��Һ��һ����