��Ŀ����
t ��ʱ����3 mol A��1 mol B����ͨ�����Ϊ2 L���ܱ�������(�ݻ�����)��������Ӧ��3A(g)��B(g) xC(g)��2 minʱ��Ӧ�ﵽƽ��״̬(�¶Ȳ���)��ʣ����0.8 mol B�������C��Ũ��Ϊ0.4 mol��L��1������д���пհף�
xC(g)��2 minʱ��Ӧ�ﵽƽ��״̬(�¶Ȳ���)��ʣ����0.8 mol B�������C��Ũ��Ϊ0.4 mol��L��1������д���пհף�
(1)�ӿ�ʼ��Ӧ���ﵽƽ��״̬������C��ƽ����Ӧ����Ϊ________��
(2)x��________��ƽ�ⳣ��K��________��
(3)��������ԭƽ�������������ͨ����������(���躤����A��B��C������Ӧ)��ѧƽ��________(��д��ĸ���)��
A��������Ӧ�����ƶ�
B�����淴Ӧ�����ƶ�
C�����ƶ�
(4)����ԭƽ��������������ٳ���a mol C����t ��ʱ�ﵽ�µ�ƽ�⣬��ʱB�����ʵ���Ϊn(B)��________mol��
(1)0.2 mol��L��1��min��1��(2)4��0.037
(3)C��(4)(0.8��0.2a)
����

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�һ���¶��£���һ��10 L�ܱ������з���ij���淴Ӧ����ƽ�ⳣ������ʽΪK�� ����ش��������⡣
����ش��������⡣
(1)�÷�Ӧ�Ļ�ѧ����ʽΪ__________________________________________��
���¶����ߣ�K������÷�Ӧ��________��Ӧ(����ȡ����ȡ�)��
(2)���жϸ÷�Ӧһ���ﵽƽ��״̬����________(����ĸ���)��
| A��v��(H2O)��v��(H2) |
| B�������������ƽ����Է�����������ʱ��ı� |
| C������n mol H2��ͬʱ����n mol CO |
| D�����������ʵ������ʵ�������ʱ��ı� |

(4)ʵ����t2ʱ����������1 mol H2O(g)��5 min��H2O(g)�����ʵ�����0.8 mol����5 min��H2O(g)��ƽ����Ӧ����Ϊ________��
 CH3OCH3��g��+3H2O��g�� ��H=-122��4kJ��mol��1
CH3OCH3��g��+3H2O��g�� ��H=-122��4kJ��mol��1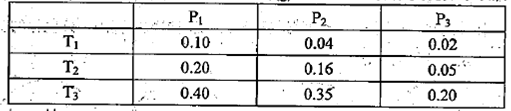
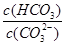 ����������䡱��С��������̼�����Һ���տ�����CO2�����ܶɳ�����ʱ�����й�ϵ��˵����ȷ���� ��
����������䡱��С��������̼�����Һ���տ�����CO2�����ܶɳ�����ʱ�����й�ϵ��˵����ȷ���� ��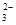 ��+c��HCO
��+c��HCO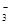 ��+c��H2CO3��
��+c��H2CO3�� c��CO
c��CO 2SO3(g�� ��H����196 kJ?mol��1����һ���̶��ݻ�Ϊ5 L���ܱ������г���0.20 mol SO2��0.10 mol O2������Ӻ�ﵽƽ�⣬��������к�SO3Ϊ0.18 mol����v(O2)���� ��mol?L��1?min��1���ų�������Ϊ�� �� kJ��
2SO3(g�� ��H����196 kJ?mol��1����һ���̶��ݻ�Ϊ5 L���ܱ������г���0.20 mol SO2��0.10 mol O2������Ӻ�ﵽƽ�⣬��������к�SO3Ϊ0.18 mol����v(O2)���� ��mol?L��1?min��1���ų�������Ϊ�� �� kJ�� 
 cC(g)��dD(g)����H��Q���Ը���ͼ�ش�
cC(g)��dD(g)����H��Q���Ը���ͼ�ش�
 CH3OCH3(g)��H2O(g) ��H����23.5 kJ��mol��1����T1�棬�����ܱ������н�������ƽ�⣬��ϵ�и����Ũ����ʱ��仯��ͼ��ʾ��
CH3OCH3(g)��H2O(g) ��H����23.5 kJ��mol��1����T1�棬�����ܱ������н�������ƽ�⣬��ϵ�и����Ũ����ʱ��仯��ͼ��ʾ��
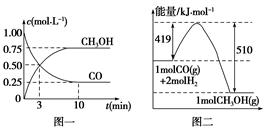
 CH3OH(g)�������ͼʾ�ش��������⣺
CH3OH(g)�������ͼʾ�ش��������⣺