��Ŀ����
����Ŀ������ѧ����ѡ��2����ѧ�뼼�������������� NaCl02����һ�ָ�Ч��������Ư������Ҫ�����ġ�ֽ��Ư�ס�ʳƷ������ˮ�����ȡ���֪��NaClO2������Һ���¶ȵ���38 ��ʱ�����ľ�����NaClO2![]() 3H2O������38 ��ʱ����������NaClO2������60��ʱNaClO2�ֽ��NaClO3��NaCl����ClO2�ֽⱬը��һ���Ʊ��������ƴֲ�Ʒ�Ĺ����������£�
3H2O������38 ��ʱ����������NaClO2������60��ʱNaClO2�ֽ��NaClO3��NaCl����ClO2�ֽⱬը��һ���Ʊ��������ƴֲ�Ʒ�Ĺ����������£�

��1��ClO2�������е����ӷ���ʽΪ ���������й��˿��������ÿ�����____��ѡ���������
a����SO2������SO3����ǿ����
b��ϡ��ClO2�Է�ֹ��ը
c����NaClO3��ԭΪClO2
��2���������ڷ�Ӧ�Ļ�ѧ����ʽΪ �����������¶Ȳ��ܳ���20������ԭ����____��
��3������ĸҺ���пɻ��յ���Ҫ������ ��
��4�����������пɻ��NaCl02��Һ����NaCl02��Һ���ֲ�Ʒ��NaClO2�������IJ�����������Ϊ������ѹ��55�������ᾧ���� ���� ��������60������õ���Ʒ��
��5��Ϊ�ⶨ��Ʒ��NaCl02������������������ʵ�飺 ȷ��ȡ��������������Ʒ10.00 g���ձ��У�������������ˮ�����ĵ⻯�ؾ��壬 �ٵ���������ϡ���ᣬ��ַ�Ӧ��ClO2-+ 4I-+ 4H+= 2H2O+ 2I2+ Cl-���������û��Һ���250mL������Һ��ȡ25.00 mL����Һ����2.000 mol��L-l Na2S203��Һ�ζ���I2+2S2O32-= 2I-+S4O62-�����������Na2SO3��Һƽ��ֵΪ16.40mL������Ʒ��NaClO2 ����������Ϊ ��
���𰸡���1��2ClO3-��SO2��2ClO2����SO42- b��
��2��2NaOH+2ClO2+H2O2=2NaClO2+2H2O+O2������ֹH2O2�ֽ�
��3��Na2SO4 ��4�����ˣ�38-60����ˮϴ�� ��5��74.21%
��������
�����������1�� ClO2�������������ơ�����������������ԭ��Ӧ���ɶ������ȣ����ӷ���ʽΪ2ClO3-��SO2��2ClO2����SO42-����ClO2�ֽⱬը���������й��˿�����������ϡ��ClO2�Է�ֹ��ը��
��2����������ClO2��������⡢�������Ʒ�Ӧ�����������ƵĻ�ѧ����ʽΪ2NaOH+2ClO2+H2O2=2NaClO2+2H2O+O2����Ϊ��ֹH2O2�ֽ⣬���������¶Ȳ��ܳ���20����
��3�� ClO2�������з�Ӧ���ɶ������ȡ������ƣ�����ĸҺ���пɻ��յ���Ҫ������Na2SO4��
��4�����������пɻ��NaCl O 2��Һ����NaCl O 2��Һ���ֲ�Ʒ��NaClO2�������IJ�����������Ϊ������ѹ��55�������ᾧ�������ˣ���38-60����ˮϴ�ӣ�������60������õ���Ʒ��
��5������Ʒ��NaClO2 xg
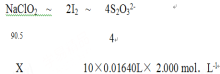
x=7.421g
����Ʒ��NaClO2����������Ϊ![]() 74.21%
74.21%

����Ŀ��ij��ѧ��ȤС��������ͼװ�ý������������ϳɺͷ����ʵ��̽������ش���������

��1��д���ϳ����������Ļ�ѧ����ʽ____________________________________��
��2������b������________��ͼ���������������õ���______����ᡢ�⡢�㡢�䡢e����
��3��Ϊ��������������IJ��ʿɲ�ȡ�Ĵ�ʩ ___________________________________
��4������0.5h���ȷ�Ӧ����Ӧװ��c�дֲ�Ʒת����d�н�������
���� | 98.3%Ũ���� | �������� | ���� | �Ҵ� | ���� | ˮ |
�е� | 338���� | 77.1�� | 118�� | 78.5�� | 34.6�� | 100�� |
�����ϱ������������õ������������У����п��ܺ���________________���ʡ�