��Ŀ����
�±���Ԫ�����ڱ���һ���֣��ش������й����⣺
��1��д������Ԫ�ط��ţ���______����______
��2��������ԭ�ӵĽṹʾ��ͼ��______�ۺ͢�Ԫ���γɵĻ������к�______������д��ѧ�����ͣ�
��3���ڢ�---���Ԫ���У���������ǿ��Ԫ����______���ǽ�������ǿ��Ԫ����______������õ�Ԫ����______��������Ԫ�ط��ű�ʾ��
��4���������������Ӧ��ˮ���ﻯѧʽ��______��
��5��Ԫ�آ���Ԫ�آ���ȣ��ǽ����Խ�ǿ����______����Ԫ�ط��ű�ʾ������һ��ѧ��Ӧ����ʽ������һ��ʵ______��
�� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | �� |
| 2 | �� | �� | ||||||
| 3 | �� | �� | �� | �� | �� | �� | �� |
��2��������ԭ�ӵĽṹʾ��ͼ��______�ۺ͢�Ԫ���γɵĻ������к�______������д��ѧ�����ͣ�
��3���ڢ�---���Ԫ���У���������ǿ��Ԫ����______���ǽ�������ǿ��Ԫ����______������õ�Ԫ����______��������Ԫ�ط��ű�ʾ��
��4���������������Ӧ��ˮ���ﻯѧʽ��______��
��5��Ԫ�آ���Ԫ�آ���ȣ��ǽ����Խ�ǿ����______����Ԫ�ط��ű�ʾ������һ��ѧ��Ӧ����ʽ������һ��ʵ______��
��Ԫ�������ڱ��е�λ�ÿ�֪����ΪN����ΪF����ΪNa����ΪMg����ΪAl����ΪSi����ΪS����ΪCl����ΪAr��
��1����ΪN����ΪF���ʴ�Ϊ��N��F��
��2����ԭ�Ӻ������Ϊ17����3�����Ӳ㣬���������Ϊ2��8��7��ԭ�ӽṹʾ��ͼΪ��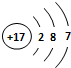 ���Ȼ����������ӻ�����������Ӽ���
���Ȼ����������ӻ�����������Ӽ���
�ʴ�Ϊ�� �����Ӽ���
�����Ӽ���
��3������ͬ����������ҽ����Լ������ǽ�������ǿ��ͬ�������϶��½�������ǿ���ǽ����Լ������ʱ��н�������ǿΪNa���ǽ�������ǿ��ΪF��ϡ������ԭ�������Ϊ�ȶ��ṹ����ѧ���������ΪAr��
�ʴ�Ϊ��Na��F��Ar��
��4��ClԪ���������Ϊ+7������������Ӧˮ����Ϊ�����ᣬ�仯ѧʽΪ��HClO4���ʴ�Ϊ��HClO4��
��5������������ҷǽ�������ǿ����ClԪ�طǽ����Ա�Sǿ������ʽNa2S+Cl2=2NaCl+S��������֤����ʵ��
�ʴ�Ϊ��Cl��Na2S+Cl2=2NaCl+S��
��1����ΪN����ΪF���ʴ�Ϊ��N��F��
��2����ԭ�Ӻ������Ϊ17����3�����Ӳ㣬���������Ϊ2��8��7��ԭ�ӽṹʾ��ͼΪ��
 ���Ȼ����������ӻ�����������Ӽ���
���Ȼ����������ӻ�����������Ӽ����ʴ�Ϊ��
 �����Ӽ���
�����Ӽ�����3������ͬ����������ҽ����Լ������ǽ�������ǿ��ͬ�������϶��½�������ǿ���ǽ����Լ������ʱ��н�������ǿΪNa���ǽ�������ǿ��ΪF��ϡ������ԭ�������Ϊ�ȶ��ṹ����ѧ���������ΪAr��
�ʴ�Ϊ��Na��F��Ar��
��4��ClԪ���������Ϊ+7������������Ӧˮ����Ϊ�����ᣬ�仯ѧʽΪ��HClO4���ʴ�Ϊ��HClO4��
��5������������ҷǽ�������ǿ����ClԪ�طǽ����Ա�Sǿ������ʽNa2S+Cl2=2NaCl+S��������֤����ʵ��
�ʴ�Ϊ��Cl��Na2S+Cl2=2NaCl+S��

��ϰ��ϵ�д�
�����Ŀ