��Ŀ����
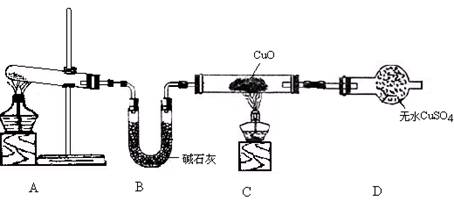
ʵ��������ͼ��ʾ�����̽�����������ʵ�飮ͼ���ü�ͷ��ʾ���������AΪһ�ִ�������������壬B����һ�����壬�����к���ɫ������֣�ʵ�������õ�ҩƷֻ�ܴ�����������ѡȡ��Na2CO3��NaHCO3��Na2O��Na2O2��NaCl����ˮCaCl2����NH4��2CO3����ʯ�ҵȹ��������ˮ��

����ͼ��װ�ú�����ش�
��1�����з�����Ӧ�Ļ�ѧ����ʽΪ______��
��2������Ӧѡ�õĸ������______��Ϊʲô��ѡ����������һ�ָ����______��
��3�����з�����Ӧ�Ļ�ѧ����ʽ______��
��4�����з�������Ҫ��Ӧ�Ļ�ѧ����ʽ______���˷�Ӧ�����ȷ�Ӧ���Ƿ��ȷ�Ӧ______�����ƿɿ���ʲô��������˵������ж�______��
�⣺���������к���ɫ������������ں��Ȳ�˿������A��B�����������ɵģ�������ѧ֪ʶ��֪�������з����ķ�ӦΪ4NH3+5O2 4NO+6H2O��A��BΪNH3��O2��������ҩƷ֪���������Ʊ�����NH3���䷴ӦΪ����NH4��2CO3
4NO+6H2O��A��BΪNH3��O2��������ҩƷ֪���������Ʊ�����NH3���䷴ӦΪ����NH4��2CO3 2NH3��+CO2��+H2O���������Ʊ�O2���䷴ӦΪ2Na2O2+2H2O=4NaOH+O2�������еĸ�����Ǽ�ʯ�ң��������ﰱ����ͬʱ��ȥCO2������ˮ�Ȼ��Ʋ�������CO2��
2NH3��+CO2��+H2O���������Ʊ�O2���䷴ӦΪ2Na2O2+2H2O=4NaOH+O2�������еĸ�����Ǽ�ʯ�ң��������ﰱ����ͬʱ��ȥCO2������ˮ�Ȼ��Ʋ�������CO2��
��1�������Ʊ�����NH3����������Լ����Եõ��Ƶİ�����Ҫ̼��泥����з�����Ӧ�Ǽ���̼��立ֽ����ɰ����Ͷ�����̼��ˮ����Ӧ�Ļ�ѧ����ʽΪ����NH4��2CO3 2NH3��+CO2��+H2O���ʴ�Ϊ����NH4��2CO3
2NH3��+CO2��+H2O���ʴ�Ϊ����NH4��2CO3 2NH3��+CO2��+H2O��
2NH3��+CO2��+H2O��
��2���Ʊ�������ʵ���Ǽ���̼��立ֽ����ɣ����еĸ�����Ǽ�ʯ�ң��������ﰱ����ͬʱ��ȥCO2������ˮ�Ȼ��Ʋ�������CO2������ʹ������ȫ������
�ʴ�Ϊ����ʯ�ң���Ϊ��һ�ָ��������ˮCaCl2��ֻ������ˮ����������CO2������ʹ������ȫ������
��3���������Ʊ�������װ�ã�����װ�ÿ�֪������Һ������Թ��й��巢����Ӧ���ɣ������ṩ�Լ��ǹ������ƺ�ˮ��Ӧ����������������Ӧ�Ļ�ѧ����ʽ��2Na2O2+2H2O=4NaOH+O2�����ʴ�Ϊ��2Na2O2+2H2O=4NaOH+O2����
��4�������з����ķ�ӦΪ4NH3+5O2 4NO+6H2O����Ӧ�Ƿ��ȷ�Ӧ��������ʵ������й۲쵽��˿���ֺ��ȣ�
4NO+6H2O����Ӧ�Ƿ��ȷ�Ӧ��������ʵ������й۲쵽��˿���ֺ��ȣ�
�ʴ�Ϊ��4NH3+5O2 4NO+6H2O�����ȷ�Ӧ�����Կ������в�˿�ȿ�ʼ��Ӧʱ���Ӻ��ȣ�
4NO+6H2O�����ȷ�Ӧ�����Կ������в�˿�ȿ�ʼ��Ӧʱ���Ӻ��ȣ�
���������������к���ɫ������������ں��Ȳ�˿������A��B�����������ɵģ�������ѧ֪ʶ��֪�������з����ķ�ӦΪ4NH3+5O2 4NO+6H2O��A��BΪNH3��O2��������ҩƷ֪���������Ʊ�����NH3���䷴ӦΪ����NH4��2CO3
4NO+6H2O��A��BΪNH3��O2��������ҩƷ֪���������Ʊ�����NH3���䷴ӦΪ����NH4��2CO3 2NH3��+CO2��+H2O���������Ʊ�O2���䷴ӦΪ2Na2O2+2H2O=4NaOH+O2�������еĸ�����Ǽ�ʯ�ң��������ﰱ����ͬʱ��ȥCO2������ˮ�Ȼ��Ʋ�������CO2��
2NH3��+CO2��+H2O���������Ʊ�O2���䷴ӦΪ2Na2O2+2H2O=4NaOH+O2�������еĸ�����Ǽ�ʯ�ң��������ﰱ����ͬʱ��ȥCO2������ˮ�Ȼ��Ʋ�������CO2��
���������⿼����ʵ����Ʒ����������Ʊ�������ƣ��Լ�ѡ���������ʵ�ʵ����֤�����жϣ���Ŀ�Ѷ��еȣ�
 4NO+6H2O��A��BΪNH3��O2��������ҩƷ֪���������Ʊ�����NH3���䷴ӦΪ����NH4��2CO3
4NO+6H2O��A��BΪNH3��O2��������ҩƷ֪���������Ʊ�����NH3���䷴ӦΪ����NH4��2CO3 2NH3��+CO2��+H2O���������Ʊ�O2���䷴ӦΪ2Na2O2+2H2O=4NaOH+O2�������еĸ�����Ǽ�ʯ�ң��������ﰱ����ͬʱ��ȥCO2������ˮ�Ȼ��Ʋ�������CO2��
2NH3��+CO2��+H2O���������Ʊ�O2���䷴ӦΪ2Na2O2+2H2O=4NaOH+O2�������еĸ�����Ǽ�ʯ�ң��������ﰱ����ͬʱ��ȥCO2������ˮ�Ȼ��Ʋ�������CO2����1�������Ʊ�����NH3����������Լ����Եõ��Ƶİ�����Ҫ̼��泥����з�����Ӧ�Ǽ���̼��立ֽ����ɰ����Ͷ�����̼��ˮ����Ӧ�Ļ�ѧ����ʽΪ����NH4��2CO3
 2NH3��+CO2��+H2O���ʴ�Ϊ����NH4��2CO3
2NH3��+CO2��+H2O���ʴ�Ϊ����NH4��2CO3 2NH3��+CO2��+H2O��
2NH3��+CO2��+H2O����2���Ʊ�������ʵ���Ǽ���̼��立ֽ����ɣ����еĸ�����Ǽ�ʯ�ң��������ﰱ����ͬʱ��ȥCO2������ˮ�Ȼ��Ʋ�������CO2������ʹ������ȫ������
�ʴ�Ϊ����ʯ�ң���Ϊ��һ�ָ��������ˮCaCl2��ֻ������ˮ����������CO2������ʹ������ȫ������
��3���������Ʊ�������װ�ã�����װ�ÿ�֪������Һ������Թ��й��巢����Ӧ���ɣ������ṩ�Լ��ǹ������ƺ�ˮ��Ӧ����������������Ӧ�Ļ�ѧ����ʽ��2Na2O2+2H2O=4NaOH+O2�����ʴ�Ϊ��2Na2O2+2H2O=4NaOH+O2����
��4�������з����ķ�ӦΪ4NH3+5O2
 4NO+6H2O����Ӧ�Ƿ��ȷ�Ӧ��������ʵ������й۲쵽��˿���ֺ��ȣ�
4NO+6H2O����Ӧ�Ƿ��ȷ�Ӧ��������ʵ������й۲쵽��˿���ֺ��ȣ��ʴ�Ϊ��4NH3+5O2
 4NO+6H2O�����ȷ�Ӧ�����Կ������в�˿�ȿ�ʼ��Ӧʱ���Ӻ��ȣ�
4NO+6H2O�����ȷ�Ӧ�����Կ������в�˿�ȿ�ʼ��Ӧʱ���Ӻ��ȣ����������������к���ɫ������������ں��Ȳ�˿������A��B�����������ɵģ�������ѧ֪ʶ��֪�������з����ķ�ӦΪ4NH3+5O2
 4NO+6H2O��A��BΪNH3��O2��������ҩƷ֪���������Ʊ�����NH3���䷴ӦΪ����NH4��2CO3
4NO+6H2O��A��BΪNH3��O2��������ҩƷ֪���������Ʊ�����NH3���䷴ӦΪ����NH4��2CO3 2NH3��+CO2��+H2O���������Ʊ�O2���䷴ӦΪ2Na2O2+2H2O=4NaOH+O2�������еĸ�����Ǽ�ʯ�ң��������ﰱ����ͬʱ��ȥCO2������ˮ�Ȼ��Ʋ�������CO2��
2NH3��+CO2��+H2O���������Ʊ�O2���䷴ӦΪ2Na2O2+2H2O=4NaOH+O2�������еĸ�����Ǽ�ʯ�ң��������ﰱ����ͬʱ��ȥCO2������ˮ�Ȼ��Ʋ�������CO2�����������⿼����ʵ����Ʒ����������Ʊ�������ƣ��Լ�ѡ���������ʵ�ʵ����֤�����жϣ���Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ




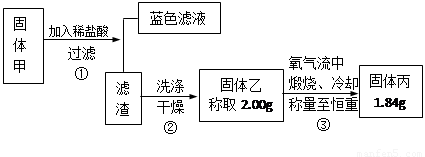
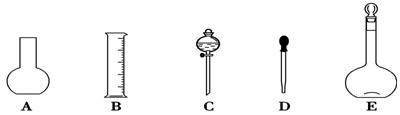
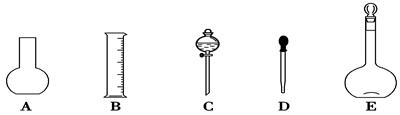
 �Σ�ϴ��ҺҲת��������ƿ
�Σ�ϴ��ҺҲת��������ƿ ������ƽ��ȡNaOH������Ϊ________g����ʵ����������������ȷ��������ʱ���ӿ̶��ߣ���������ҺŨ��________0.1 mol��L��1 (����ڡ������ڡ���С�ڡ�����ͬ)����NaOH��Һ��ת��������ƿʱ����������������������ҺŨ��________0.1 mol��L��1���ܽ�NaOH����δ��������ʱ��������N
������ƽ��ȡNaOH������Ϊ________g����ʵ����������������ȷ��������ʱ���ӿ̶��ߣ���������ҺŨ��________0.1 mol��L��1 (����ڡ������ڡ���С�ڡ�����ͬ)����NaOH��Һ��ת��������ƿʱ����������������������ҺŨ��________0.1 mol��L��1���ܽ�NaOH����δ��������ʱ��������N aOH��Һת��������ƿ���ݣ���������ҺŨ��_______ 0.1 mol��L��1��
aOH��Һת��������ƿ���ݣ���������ҺŨ��_______ 0.1 mol��L��1��