��Ŀ����
����Ŀ��ijͬѧ��ʵ�����������������NO��̽��ʵ�顣
��һ����ҵ����һ���¶Ⱥʹ�����������NH3��NOx��ԭ����N2��̽��NO��NH3�ܷ�Ӧ��
��1���������Ʊ�

�ٰ����ķ���װ�ÿ���ѡ����ͼ�е�_____����Ӧ�Ļ�ѧ����ʽ______________________
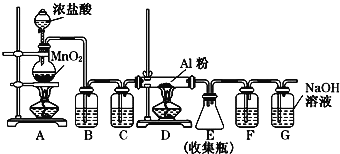
�����ռ�һƿ����İ�����ѡ����ͼ�е�װ�ã�������˳��Ϊ������װ����__________��������������Сд��ĸ��ʾ����
��2���������ռ�����NH3������������������NO����ֻ�ϣ���ȴ�����£��۲쵽����������ˮ�飬˵��NO��NH3�ܷ�Ӧ�����������ͻ�ԭ�������ʵ���֮��Ϊ__________��
������̽��һ�������ܷ�Na2O2��ȫ���գ����������ʵ�顣װ�����£�����װ��ʡ�ԣ���

����������֪����2NO+Na2O22NaNO2
�����������£�NO��NO2������KMnO4��Һ��Ӧ����NO3 -��
�ش��������⣺
��1������a���ƣ�________________ ����
��2��Bƿ��װ�������ǣ�_______________ ��
��3����NO�ܱ�Na2O2��ȫ���գ�Eװ���е�����Ϊ ______________ ��
��4��������ƿA�з�Ӧ�Ļ�ѧ����ʽΪ____________________________��
��5��Cװ�õ�������____________________________________________��
���𰸡�A����B �� 2NH4Cl +Ca(OH)2 ![]() 2NH3��+CaCl2 +2H2O����NH3��H2O
2NH3��+CaCl2 +2H2O����NH3��H2O ![]() NH3��+H2O�� d��c��f��e��i 3:2 ��Һ©�� ˮ ���Ը��������Һ����ɫ C+4HNO3��Ũ��
NH3��+H2O�� d��c��f��e��i 3:2 ��Һ©�� ˮ ���Ը��������Һ����ɫ C+4HNO3��Ũ��![]() CO2��+4NO2��+2H2O ����NO,��ȥ������̼
CO2��+4NO2��+2H2O ����NO,��ȥ������̼
��������
��һ������1������ʵ�����������ù�����ʯ�������NH4Cl��ϼ��ȣ���Ӧѡ��Aװ�ü��ȣ�������Ӧ�Ļ�ѧ����ʽΪ2NH4Cl+Ca��OH��2![]() CaCl2+2NH3��+2H2O����������NH3��H2O
CaCl2+2NH3��+2H2O����������NH3��H2O![]() NH3��+H2O��ȡNH3����Ҳ����ѡBװ�ã��ʴ�Ϊ��A����B����2NH4Cl+Ca(OH)2
NH3��+H2O��ȡNH3����Ҳ����ѡBװ�ã��ʴ�Ϊ��A����B����2NH4Cl+Ca(OH)2![]() 2NH3��+CaCl2 +2H2O����NH3��H2O
2NH3��+CaCl2 +2H2O����NH3��H2O![]() NH3��+H2O����
NH3��+H2O����
������Aװ���Ƶõİ��������ڰ����Ǽ������壬������Ҫ���ü�ʯ�ҽ��и��Ȼ���ٸ��ݰ������ܶȱȿ���С�����ʣ��������ſ������ռ��������Ǵ�����Ⱦ�Ҫ����β������������������ˮ�м������ܽ⣬��ˮ�����ռ�����β����������װ�õ�����˳��Ϊd��c��f��e��i���ʴ�Ϊ��d��c��f��e��i��
��2��NO���������ԣ�NH3�л�ԭ�ԣ������������ᷢ����Ӧ����������ˮ�����ݵ����غ㡢ԭ���غ㣬�ɵ÷�Ӧ�ķ���ʽ��4NH3+6NO=5N2+6H2O��NH3��NԪ�صĻ��ϼ���-3������Ϊ0�ۣ����ϼ����ߣ�����ԭ����NO��NԪ�صĻ��ϼ���+2�۽���Ϊ0�ۣ����ϼ۽��ͣ��������������ʵ���֮�ȵ��ڻ�ѧ������֮�ȣ����������ͻ�ԭ�������ʵ���֮��3:2���ʴ�Ϊ��3:2��
����������1�����������Ĺ����ص��֪������a�������Ƿ�Һ©�����ʴ�Ϊ����Һ©����
��2��̼��Ũ���ᷴӦ�IJ����Ƕ�����������ʵ��Ҫ̽������һ�������Ƿ�Na2O2��ȫ���գ���Ӧ�跨�ö���������ˮ��Ӧת��ΪNO�����Bƿ��װ��������ˮ���ʴ�Ϊ��ˮ��
��3����NO�ܱ�Na2O2��ȫ���գ�Eװ���е�����Ϊ���Ը��������Һ����ɫ���ʴ�Ϊ�����Ը��������Һ����ɫ��
��4��������ƿA�з�Ӧ�Ļ�ѧ����ʽΪC+4HNO3��Ũ��![]() CO2��+4NO2��+2H2O���ʴ�Ϊ��C+4HNO3��Ũ��
CO2��+4NO2��+2H2O���ʴ�Ϊ��C+4HNO3��Ũ��![]() CO2��+4NO2��+2H2O��
CO2��+4NO2��+2H2O��
��5��Cװ�õ������Ǹ���NO����ȥ������̼���ʴ�Ϊ������NO����ȥ������̼��