��Ŀ����
ԭ���������������X��Y��Z��W���ֶ�����Ԫ�أ�X��Wԭ�ӵ�����������������Ӳ� ����ȣ�Y�ֱܷ���X��Z�γ�ԭ�Ӹ�����Ϊ1:3�Ļ����������YX3��һ�ִ̼�����ζ �����塣����˵����ȷ����
| A��ԭ�Ӱ뾶��Z<Y<W�������Ӱ뾶��W<Y<Z |
| B��W���Ȼ���ˮ��Һ�еμӹ���Z�����γɵı�����Һ��������W����� |
| C����X��Y��Z����ε�ˮ��Һ�����ԣ�����Һ�и���������Ũ�ȴ����������Ũ�� |
| D����֪YX3�е�Զ����YZ3����Y��X���ļ��ܸ���Y��Z�� |
B
�����������������YX3�д̼�����ζ��ΪNH3��YZ3��Z����Ϊ±��Ԫ�أ�����X��W���������������ڵ��Ӳ�����ΪH��Al��X��Y��Z��W�ֱ���H��N��F��Al��A��ԭ�Ӱ뾶�� Al>(B)>N>F�����Ӱ뾶��N3->F->Al3+������B���Ȼ�����Һ�еμӹ���NaF������Һ��������AlF63-����ȷ��C��NH4F��Һ�����ԣ��ɵ���غ�c(NH4+)+c(H+)=c(F-)+c(OH-)��c(NH4+) <c(F-)������D��NH3�ķе�Զ����NF3����NH3�γɷ��Ӽ����������

��ϰ��ϵ�д�
�����Ŀ
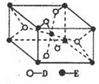

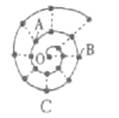
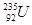 ��ԭ�Ӻ��ڵ���������
��ԭ�Ӻ��ڵ���������